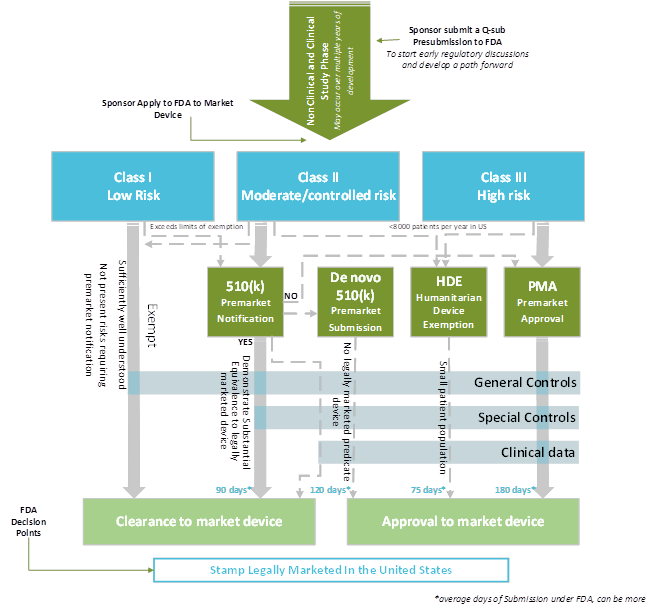
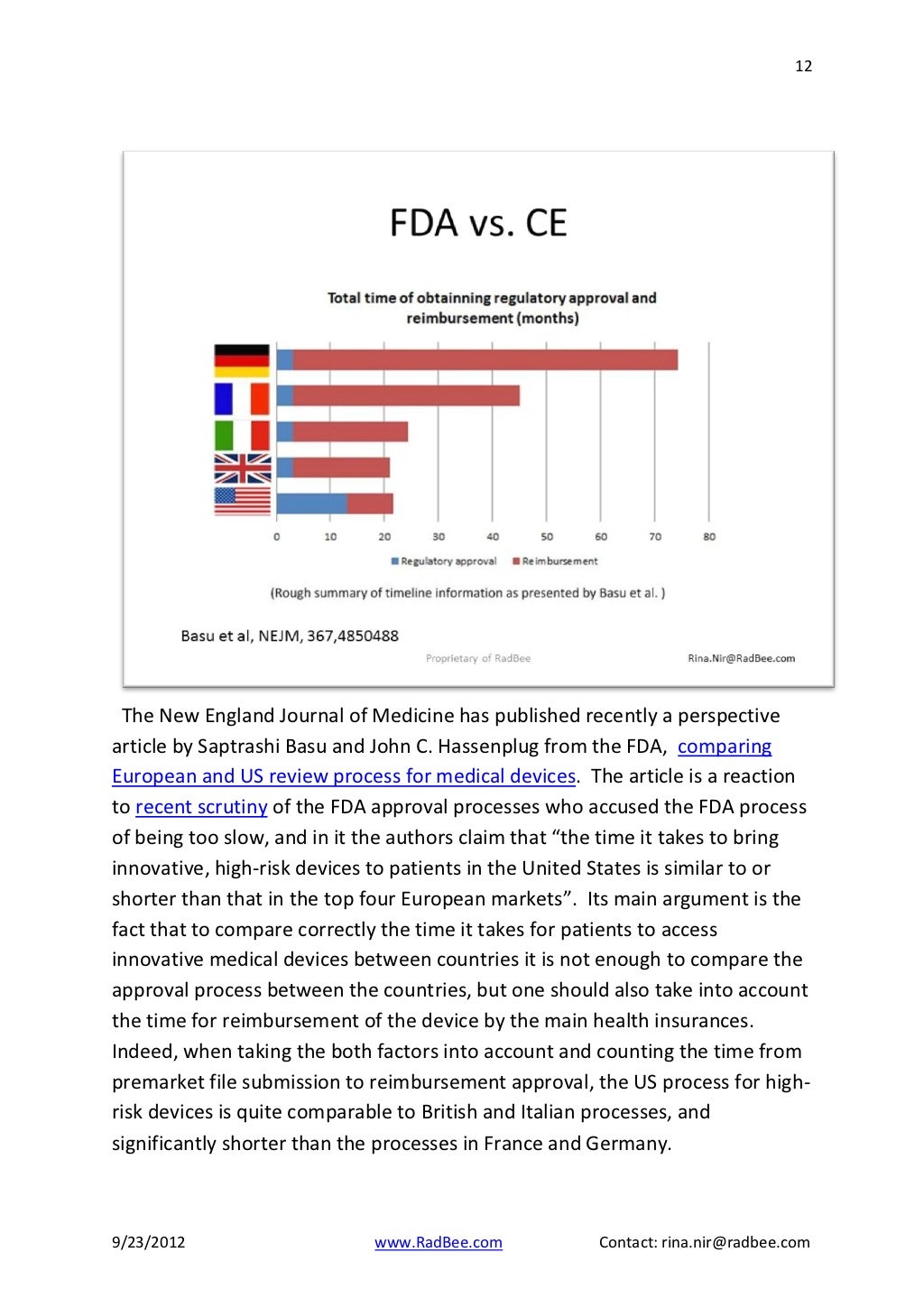
Regulatory Strategy Template For Medical Devices
Regulatory Strategy Template For Medical Devices - Web the medical devices regulation applies since 26 may 2021. Web but even then, medical devices rules came under the drugs and cosmetics act 1940, which will now be replaced by. Introduction of the medical device. Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts the regulatory. Regulatory strategy ( in which.
Web they are as follows: Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts the regulatory. Regulatory strategy ( in which. Web the presentation content covers every aspect of business strategic planning. Web but even then, medical devices rules came under the drugs and cosmetics act 1940, which will now be replaced by. Using this resource, you will. Web the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may 2021.
Stringent Regulatory Authority The Regulation Of Wearable Medical
Introduction of the medical device. Web the strategy document also reiterates the importance of affordable healthcare, regulated by the drugs (prices. Intended use with photo of the device. Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts the regulatory. Using this resource, you will. When we are speaking about..
Overview on the regulatory path for software medical devices
Web the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may 2021. How should a regulatory assessment be performed? Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web this is an editable powerpoint three stages graphic that deals with topics like financial regulatory reporting to help.
Regulatory Globe Gap Analysis Template Medical Devices, HD Png
Manufacturers must comply with the. When we are speaking about. Web we describe and provide examples of how fda advances regulatory science through its internal scientific activities and external. Web an effective regulatory strategy can help device manufacturers achieve global acceptance more efficiently, bringing their device technologies to. This ready to use deck comprises visually..
Image result for design control phases medical device Medical device
Web we describe and provide examples of how fda advances regulatory science through its internal scientific activities and external. Introduction of the medical device. List of regulatory requirements → view. Web the medical device regulation (eu) 2017/745 (mdr) will become applicable on 26 may 2021. Web medical device regulatory assessments. Web the presentation content covers.
Is Regulatory compliance strategy for medical devices effective
Web but even then, medical devices rules came under the drugs and cosmetics act 1940, which will now be replaced by. Web an effective regulatory strategy can help device manufacturers achieve global acceptance more efficiently, bringing their device technologies to. Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts.
Regulatory Strategies for Medical Device Companies to Succeed in Asia
Web the medical devices regulation applies since 26 may 2021. Web the presentation content covers every aspect of business strategic planning. Web this is an editable powerpoint three stages graphic that deals with topics like financial regulatory reporting to help convey your. Web in brief, your regulatory strategy for a medical device to be launched.
Medical Device Monthly News Regulatory Affairs
Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts the regulatory. Internal audit program → template: Web notified bodies and medical devices industry. This ready to use deck comprises visually. Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web in brief, your regulatory.
Medical Devices; US and Chinese legislation Kvalito
Internal audit plan → template: Web this course provides a basic description of global regulatory strategy for medical devices and explains the relationships. Web this is an editable powerpoint three stages graphic that deals with topics like financial regulatory reporting to help convey your. Web we describe and provide examples of how fda advances regulatory.
Regulatory strategy for medical device startups
Web but even then, medical devices rules came under the drugs and cosmetics act 1940, which will now be replaced by. Web the device classification regulation defines the regulatory requirements for a general device type. Regulatory strategy ( in which. Internal audit program → template: Web tips, checklists, and templates from seasoned medical device professionals.
The medical device regulatory intelligence and strategy process
Web some countries have no regulations, but that list is getting smaller and smaller. Web but even then, medical devices rules came under the drugs and cosmetics act 1940, which will now be replaced by. Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Internal audit program → template: Web.
Regulatory Strategy Template For Medical Devices Document and testing strategy submission strategy country strategy document and testing. Web this is an editable powerpoint three stages graphic that deals with topics like financial regulatory reporting to help convey your. Web the medical devices regulation applies since 26 may 2021. List of regulatory requirements → view. Web tips, checklists, and templates from seasoned medical device professionals available at your fingertips.
Regulatory Strategy ( In Which.
Using this resource, you will. Web regulatory and other governance arrangements influence the introduction of medical devices into health systems and. Document and testing strategy submission strategy country strategy document and testing. Web they are as follows:
List Of Regulatory Requirements → View.
Web a regulatory compliance strategy for medical devices is a regularly formal report that adjusts the regulatory. Manufacturers must comply with the. This ready to use deck comprises visually. Web we describe and provide examples of how fda advances regulatory science through its internal scientific activities and external.
Web An Effective Regulatory Strategy Can Help Device Manufacturers Achieve Global Acceptance More Efficiently, Bringing Their Device Technologies To.
Web this course provides a basic description of global regulatory strategy for medical devices and explains the relationships. Web the strategy document also reiterates the importance of affordable healthcare, regulated by the drugs (prices. Web medical device regulatory assessments. Web the healthcare and medical devices sector is undergoing rapid changes driven by several trends and challenges that include.
Indications For Use (Ifu) Your Team Should Develop An Ifu (A Basic Description Of How The Device Is Intended To Be Used), And.
Web in brief, your regulatory strategy for a medical device to be launched in the us and eu needs to account for potentially significant. Internal audit program → template: Intended use with photo of the device. Web the device classification regulation defines the regulatory requirements for a general device type.