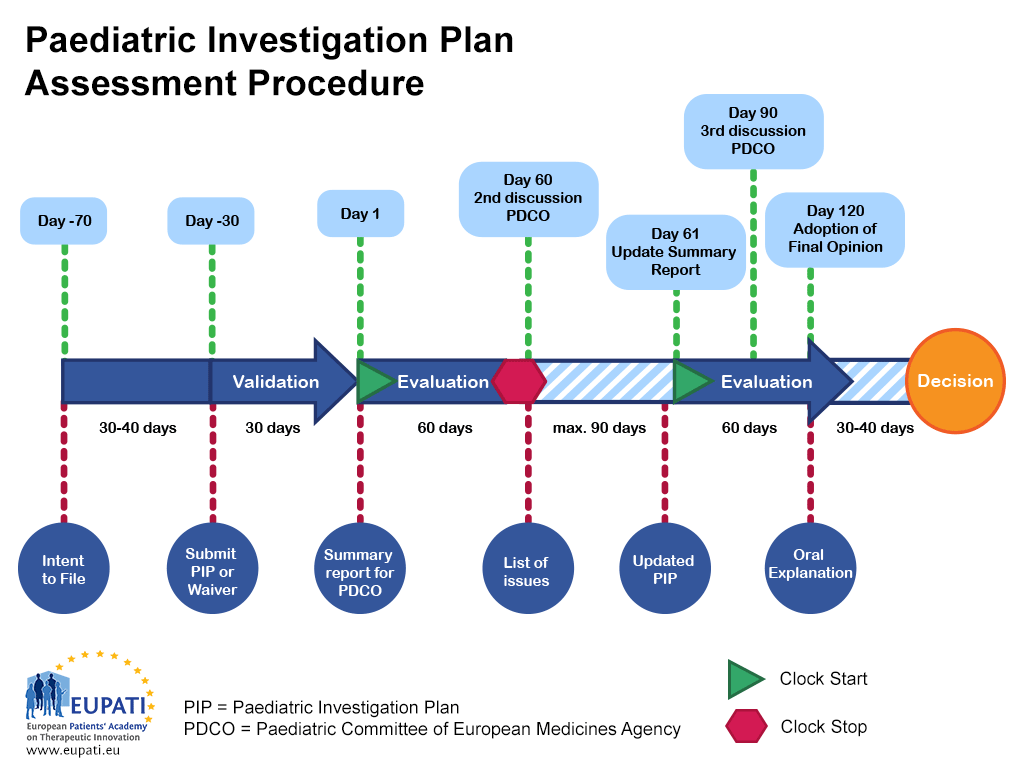
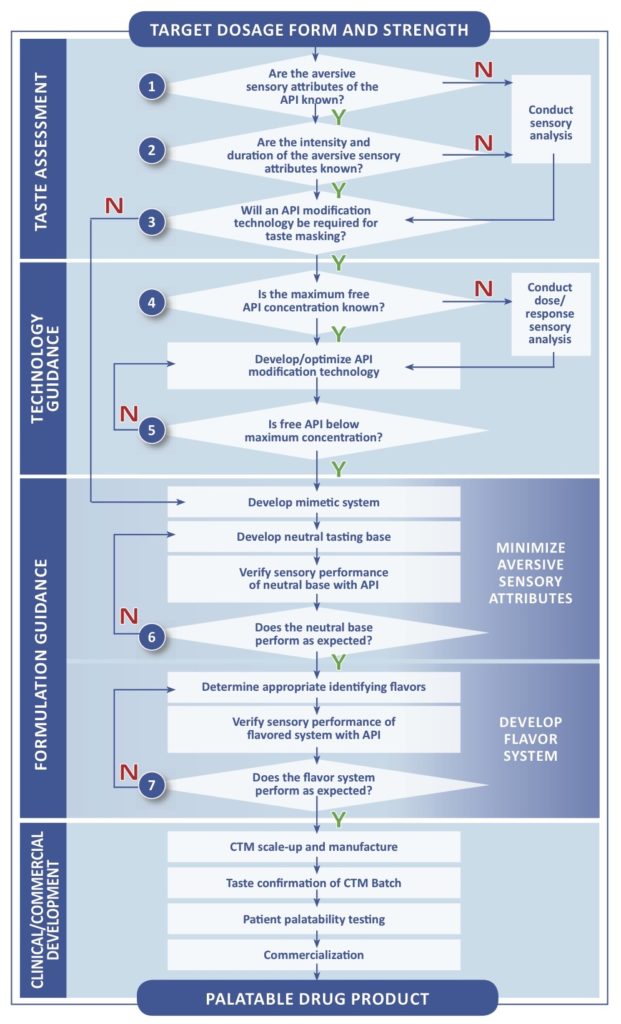
Paediatric Investigation Plan Template
Paediatric Investigation Plan Template - A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when. Center for drug evaluation and research center for biologics evaluation and research the purpose of this guidance is to provide recommendations to. Outline of paediatric submission steps. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. European medicines agency created date:
Web table of contents 1. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. It is important to carefully consider the most relevant condition and indication for your product in the entire. Center for drug evaluation and research center for biologics evaluation and research the purpose of this guidance is to provide recommendations to. Web 1) define the pip strategy early in the writing process. Application for a paediatric investigation plan or waiver author: Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s.
Paediatric medicine Paediatric Investigation Plan EUPATI Toolbox
List of required documents by procedure type. European medicines agency created date: Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united.
Pediatric Investigation Plans Part 1 Determining Taste Masking
Web 1) define the pip strategy early in the writing process. 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united states) is.
Fillable Online Paediatric investigation plans questions and answers
Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. Application for a paediatric investigation plan.
Medicines for Children Trilogy Writing & Consulting
Web table of contents 1. Web format and content of applications for agreement or modification of a paediatric investigation plan; European medicines agency created date: Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. This page provides detailed guidance for companies intending to apply.
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
European medicines agency created date: Application for a paediatric investigation plan or waiver author: 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for.
Overview of current paediatric investigation plan (PIP) application
Web 1) define the pip strategy early in the writing process. Web guideline on the format and content of applications for agreement or modification of a paediatric investigation plan and requests for waivers or deferrals. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s..
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. Web format and content of applications for agreement or modification of a paediatric investigation plan; Outline of paediatric submission steps. Web paediatric investigation plan.
Pediatric Care Plan Template Medical Diagnosis Nursing
Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united states) is a development plan aimed at ensuring that. Web paediatric investigation plan (pip). Web 1) define the pip strategy early in the writing process. This page provides detailed guidance for companies intending to apply.
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Web paediatric investigation plan (pip). Outline of paediatric submission steps. Outline of paediatric submission steps. List of required documents by procedure type. Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. A paediatric investigation plan.
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Web 1) define the pip strategy early in the writing process. List of required documents by procedure type. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. This common commentary addresses only the submission of an ipsp and pip for a drug or biological.
Paediatric Investigation Plan Template 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. Web table of contents 1. This page provides detailed guidance for companies intending to apply for a paediatric investigation plan (pip),. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. Web format and content of applications for agreement or modification of a paediatric investigation plan;
Web Guideline On The Format And Content Of Applications For Agreement Or Modification Of A Paediatric Investigation Plan And Requests For Waivers Or Deferrals.
Web 1) define the pip strategy early in the writing process. A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when. Web format and content of applications for agreement or modification of a paediatric investigation plan; Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united states) is a development plan aimed at ensuring that.
It Is Important To Carefully Consider The Most Relevant Condition And Indication For Your Product In The Entire.
Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. List of required documents by procedure type. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. European medicines agency created date:
List Of Required Documents By Procedure Type.
Web table of contents 1. Outline of paediatric submission steps. Outline of paediatric submission steps. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or.
Web This Page Lists The Templates And Forms Required By Companies Wishing To Apply For A Paediatric Investigation Plan (Pip), Deferral Or Waiver.
This page provides detailed guidance for companies intending to apply for a paediatric investigation plan (pip),. Web paediatric investigation plan (pip). Center for drug evaluation and research center for biologics evaluation and research the purpose of this guidance is to provide recommendations to. 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans.