How To Draw Ionic Bonds
How To Draw Ionic Bonds - Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web the two types of bonding are covalent, for the sharing of electrons between atoms, and ionic, for the net transfer of electrons between atoms. Web 6.7k views 7 years ago edexcel chemistry 2022: Magnesium now has an empty third shell so there is no need to draw a third. Covalent or ionic bonding will determine the type of compound that will be formed.
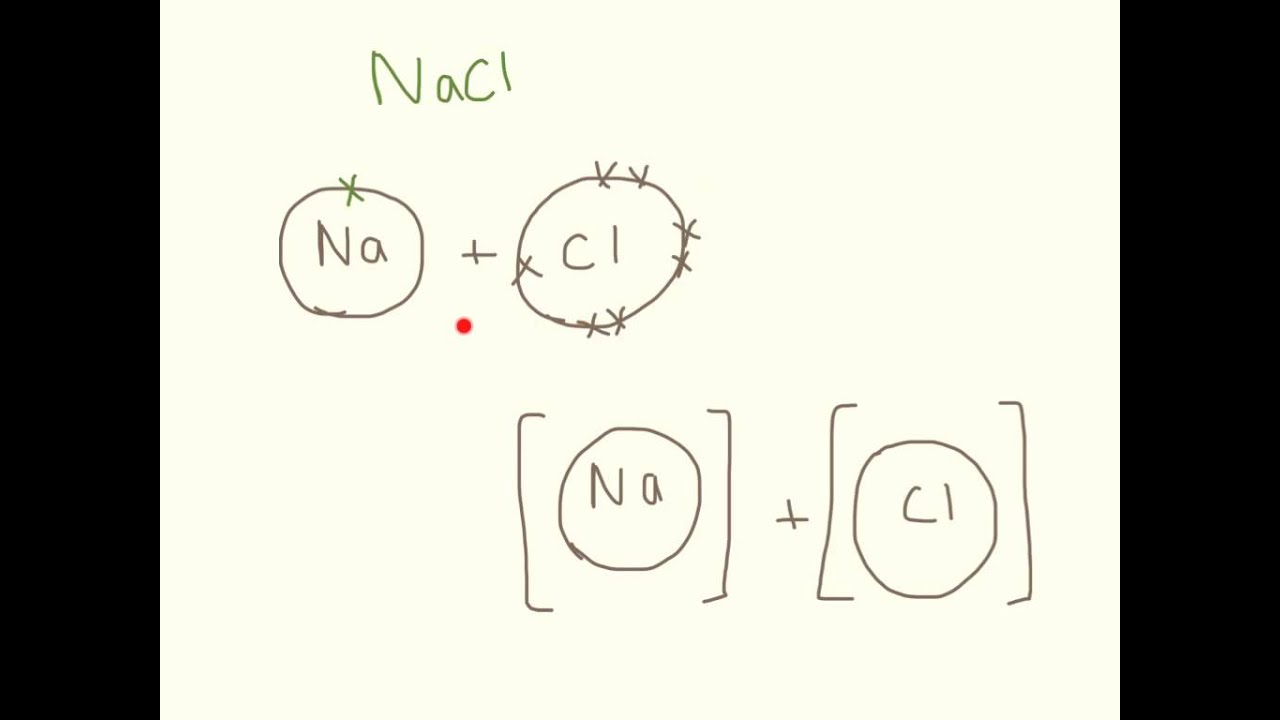
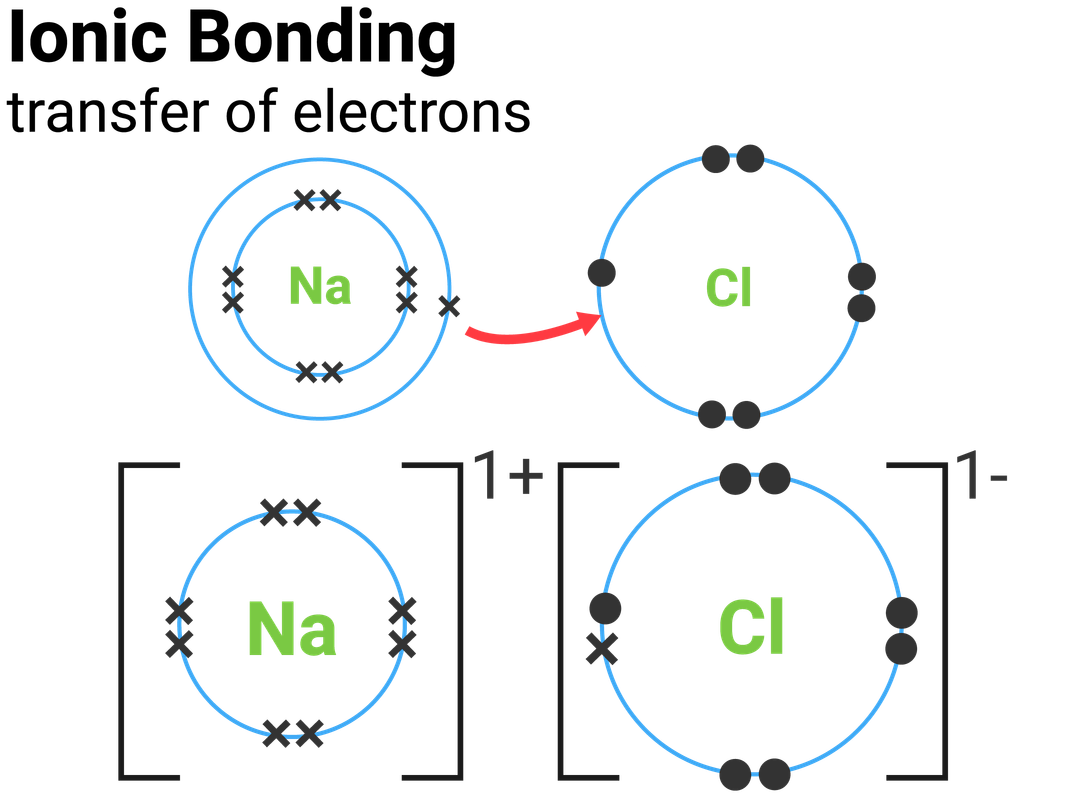
Instead ionic compounds stick together through electrostatic forces (different electrically charged ions) which we usually represent with brackets and the charge in the upper right corner. Web in ionic bonding, atoms transfer electrons to each other. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Web representing a covalent bond using lewis structures. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Even if you don't want to stud. Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds.
Lewis Structure Of Ionic Compounds
In a covalent bond, the electrons are shared between atoms. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Web once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and.
drawing ionic bonds worksheet lineartdrawingsheartflowers
Web representing a covalent bond using lewis structures. In chapter 1, we used atomic theory to describe the structure of the fluorine atom. Web swap the crosses for dots in one of your diagrams. Even if you don't want to stud. Web to begin our exploration of bonding, we need to define the two main.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. In chapter 1, we used atomic theory to describe the structure of the fluorine atom. Ionic bonds require at least one electron donor and one electron acceptor. Covalent or ionic bonding will determine the type of compound that will.
6 ionic bonding 01 — Postimages
Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. Drawing lewis structures for molecules with one central atom: If it could gain an electron from somewhere it too would become more stable. Web it's just for ionic compounds electrons aren't shared so you.
subatomic particles Montessori Muddle
Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds. Web ionic bonding in sodium chloride. It is assumed that only electrons in the valence shell are involved in the formation of covalent bonds. In a covalent bond, the electrons.
How Ions Are Formed? Infrared for Health
Web once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Web it's just for ionic compounds electrons aren't shared so you won't have things like single bonds between atoms. Ionic bonds require at least one electron donor and one.
Ionic Bonding Diagram
Examples include nacl, mgf2, k2o, and al2o3. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. 1.21b explain how to draw ionic bonding.more. Covalent or ionic bonding will determine the type of compound that will.
2016 topic 4.1 bonding ionic
Ionic bonds require at least one electron donor and one electron acceptor. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web this crash course chemistry video tutorial explains the main concepts between ionic bonds found.
How Do Ions Increase Conductivity? Atlas Scientific
These grades are the stepping stone to your future. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Web the two types of bonding are covalent, for the sharing of electrons between atoms, and ionic, for the net transfer of electrons between atoms. Ionic bonds require at least.
How to draw ionic bonding dot and cross diagrams Feature RSC Education
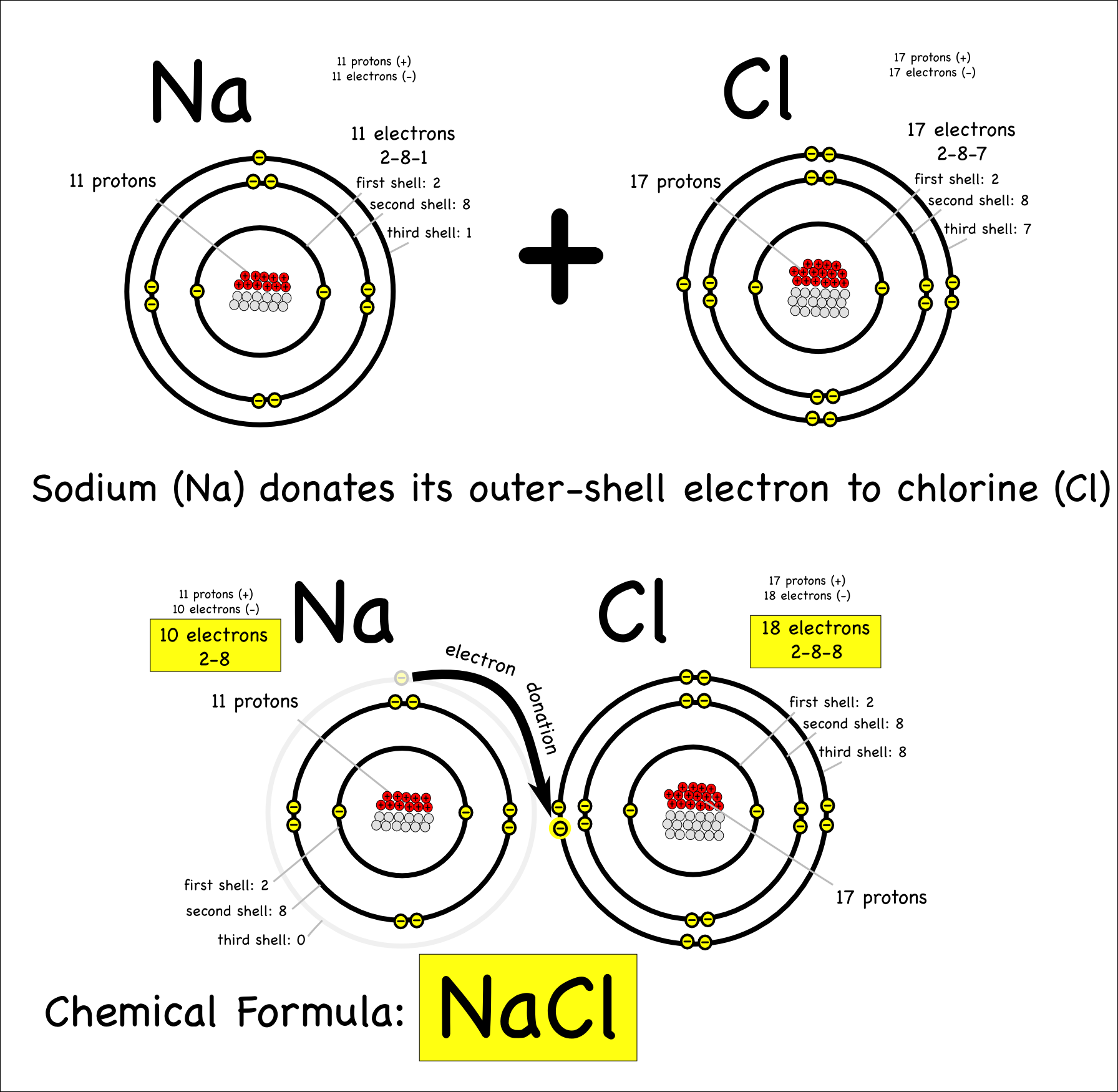
Web draw a lewis electron dot diagram for an atom or a monatomic ion. 1.7.1 formation of covalent bonds; Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. Web ionic bonding in sodium chloride. The two electrons in the bond are simultaneously attracted.
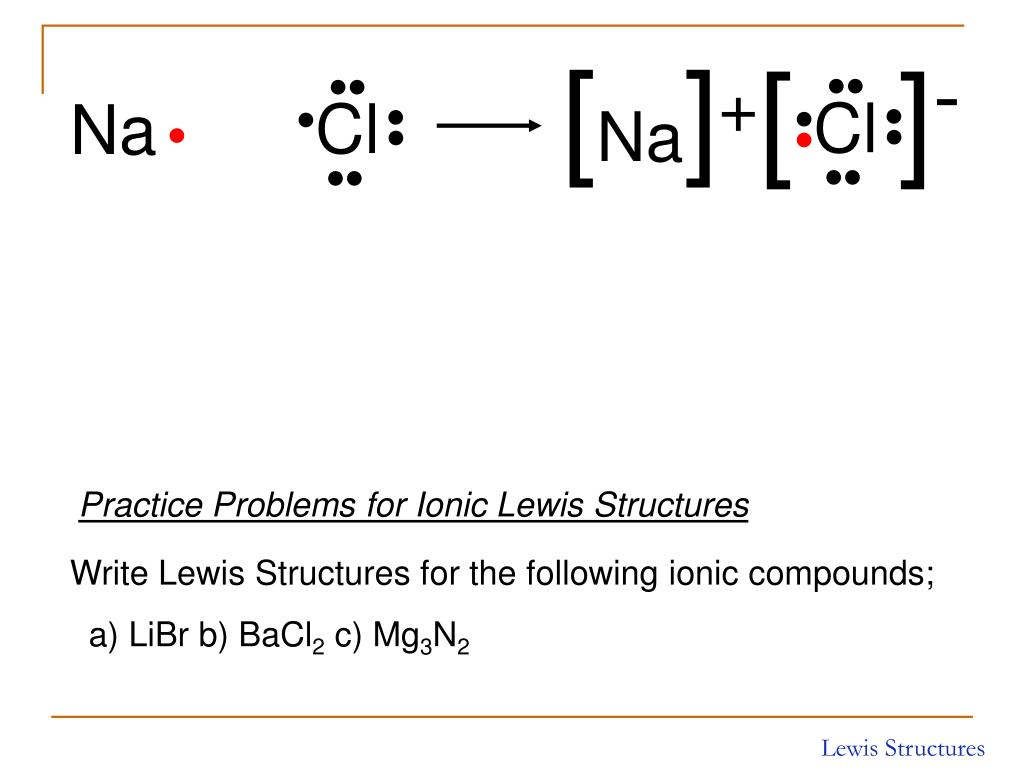
How To Draw Ionic Bonds Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. 1.21b explain how to draw ionic bonding.more. An ionic compound is made up of charged particles, called ions. Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. Examples include nacl, mgf2, k2o, and al2o3.
The Two Electrons In The Bond Are Simultaneously Attracted To Both Nuclei.
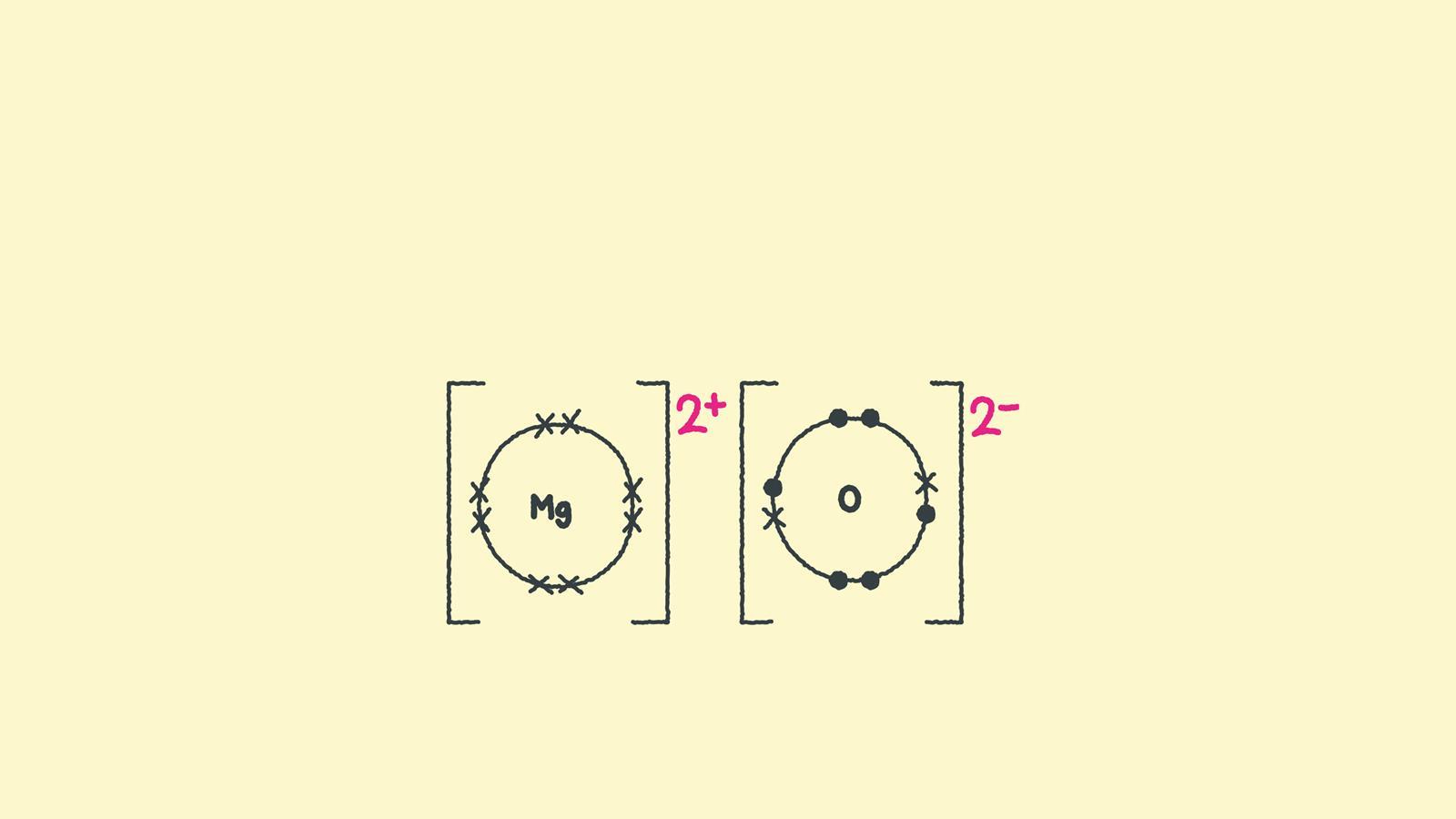
These grades are the stepping stone to your future. Web ionic bonding the atoms form ions by gaining or losing electrons to obtain electron configurations with a full outer shell. Shows how to draw lewis dot structures for ionic compounds. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
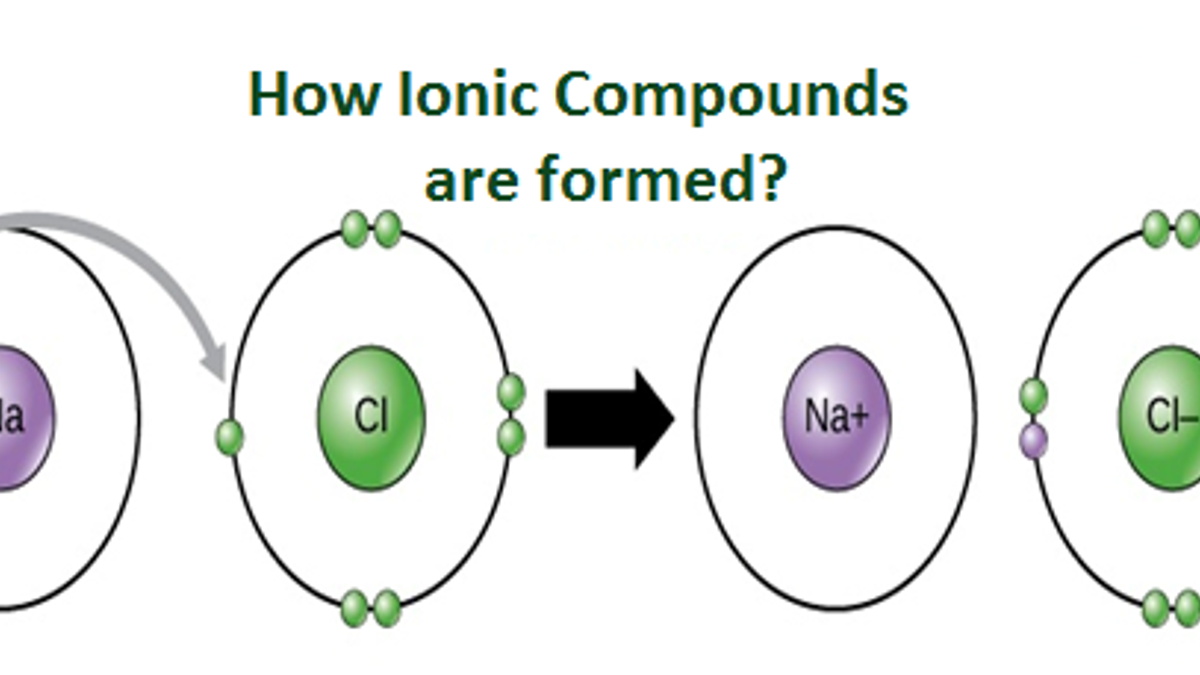
Sodium (2,8,1) Has 1 Electron More Than A Stable Noble Gas Structure (2,8).
Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds. If it gave away that electron it would become more stable. Web for exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. In electron transfer, the number of electrons lost must equal the number of electrons gained.
Examples Include Nacl, Mgf2, K2O, And Al2O3.
The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Web to begin our exploration of bonding, we need to define the two main types of bonds: Web draw a lewis electron dot diagram for an atom or a monatomic ion.
In A Covalent Bond, The Electrons Are Shared Between Atoms.
In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. Ionic bonds require at least one electron donor and one electron acceptor. Covalent or ionic bonding will determine the type of compound that will be formed. Web the two types of bonding are covalent, for the sharing of electrons between atoms, and ionic, for the net transfer of electrons between atoms.