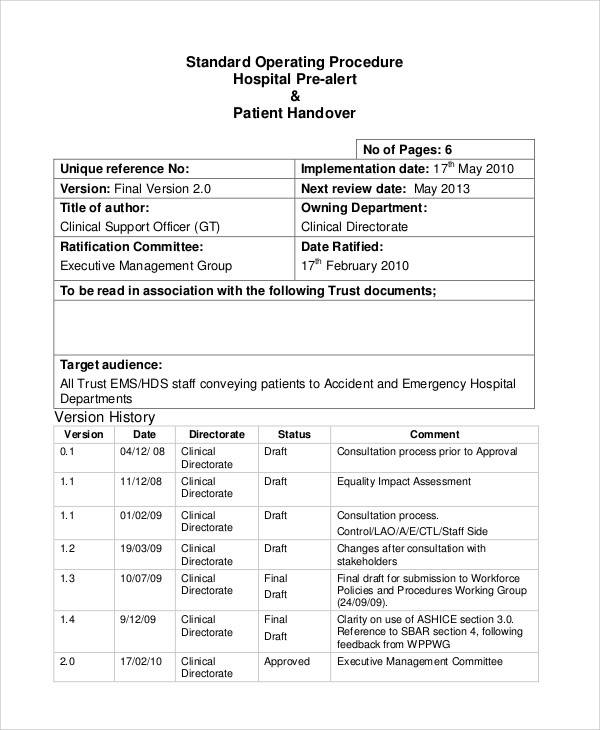
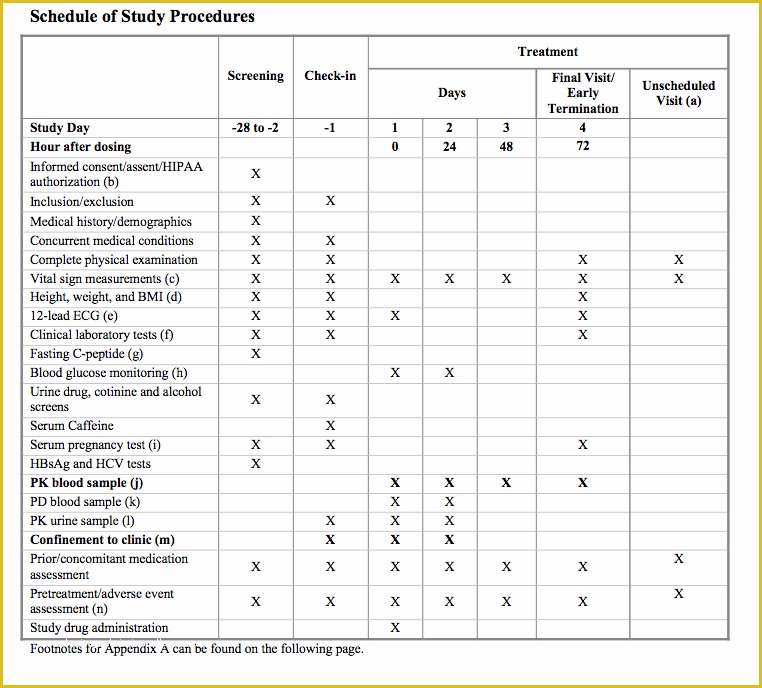
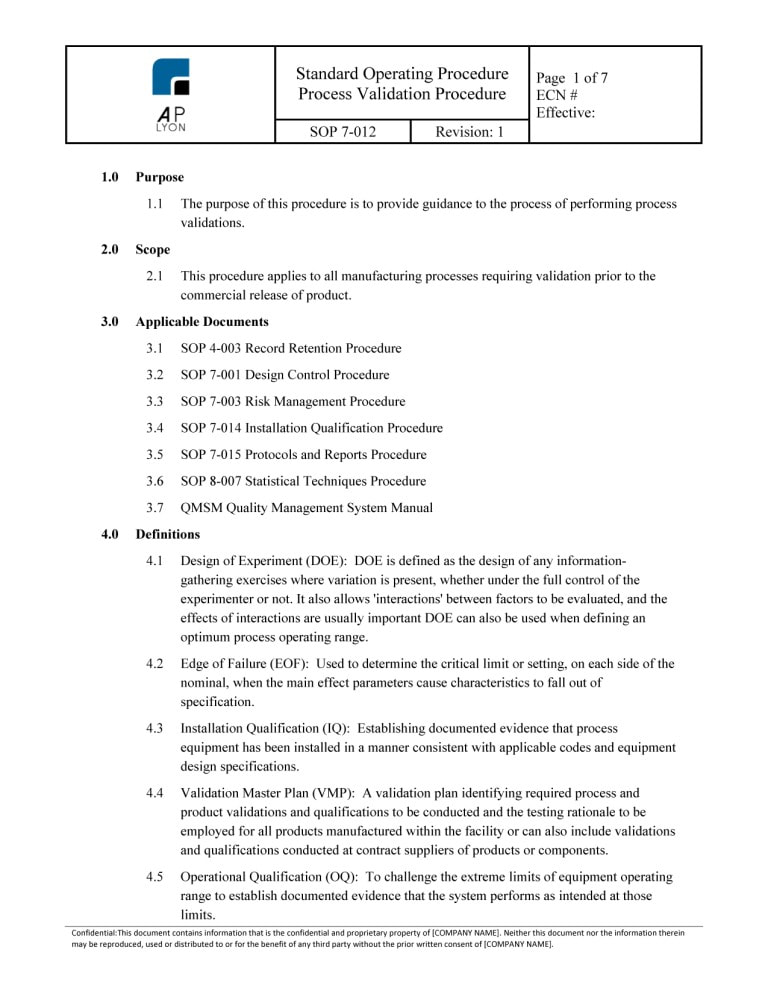
Fda Sop Template
Fda Sop Template - Outlines the scope of the validation. Generally accepted accounting principles (gaap); Intake form for fda/ohrp inspection requests. International financial reporting standards (ifrs) Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to.
Web the compliance monitoring team has created standard operating procedure templates (sops) in. Web food safety plan & haccp templates. Are designed to get your project moving quickly and efficiently. Web fda inspection information. Web download software quality assurance sops and computer system validation templates. Web complete fda sop template online with us legal forms. Generally accepted accounting principles (gaap);
PRODUCT RECALLS SOP Template MD32 GMP, QSR & ISO Compliance
These sop's will provide a foundation for. Are designed to get your project moving quickly and efficiently. Web inspection preparedness standard operating procedure template sop number: Use sops to show compliance with some of the following standards and regulations: Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. Gain the knowledge and tools.
Change Control Management SOP.pdf Food And Drug Administration
Use sops to show compliance with some of the following standards and regulations: Web the compliance monitoring team has created standard operating procedure templates (sops) in. Web fda sends warning letters to companies for not having adequate sops, as well as not following them. Purpose/policy this document describes the medical device single audit program. Web.
FREE 35+ SOP Templates in PDF
Web sops and sop templates are also common in government and military organizations. Intake form for fda/ohrp inspection requests. Purpose/policy this document describes the medical device single audit program. These sop's will provide a foundation for. Generally accepted accounting principles (gaap); International financial reporting standards (ifrs) Save or instantly send your. Web inspection preparedness standard.
SOP For Corrective Action and Preventive Actions Pharmaceutical
Web methods, templates, policies, regulations, etc. Web alvin tai wanted to help companies comply with fda and iso standards, so he started releasing free. Web fda sends warning letters to companies for not having adequate sops, as well as not following them. These sop's will provide a foundation for. Web sops and sop templates are.
FDA statement Citinewsroom Comprehensive News in Ghana
Food and drug administration office of regulatory affairs. Web sops and sop templates are also common in government and military organizations. Web procedures describe who, what, when, and how. Web inspection preparedness standard operating procedure template sop number: Web food safety plan & haccp templates. Web complete fda sop template online with us legal forms..
Clinical Research sop Template Free Of Ich Gcp E6 R2 Addendum Risk
Any optional section that does not have content will be annotated. Web complete fda sop template online with us legal forms. Easily fill out pdf blank, edit, and sign them. Web procedures describe who, what, when, and how. The validation center™ library has the. Web this manual of policies and procedures (mapp) specifies the factors.
Operating Procedure Example Sample Templates Standard FDA Sop inside
Web sops and sop templates are also common in government and military organizations. Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to. Web the compliance monitoring team has created standard operating procedure templates (sops) in. Food and drug administration office of regulatory affairs. Web download software quality.
Medical Device Process Validation Procedure ISO 13485 and FDA QSR
Web fda inspection information. Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. Gain the knowledge and tools to assess your existing sops for the most common issues. Web fda sends warning letters to companies for not having adequate sops, as well as not following them. These sop's will provide a foundation for..
Validation Quality Plan FDA EU WHO cGMP QbD SOP's
Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to. Food and drug administration office of regulatory affairs. Web alvin tai wanted to help companies comply with fda and iso standards, so he started releasing free. Web the compliance monitoring team has created standard operating procedure templates (sops).
PHARMACY Standard Operating Procedure (SOP) for Pharmacy
Save or instantly send your. Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. Use sops to show compliance with some of the following standards and regulations: Web food safety plan & haccp templates. Web sops and sop templates are also common in government and military organizations. Web the compliance monitoring team has.
Fda Sop Template International financial reporting standards (ifrs) Generally accepted accounting principles (gaap); Outlines the scope of the validation. Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to. Web sops and sop templates are also common in government and military organizations.
Web Viewed 119 Times 0 Is There A Template That Could Help Me Formatting Look Like An Official Fda Sop?
Web sops and sop templates are also common in government and military organizations. Web procedures describe who, what, when, and how. These sop's will provide a foundation for. Web the compliance monitoring team has created standard operating procedure templates (sops) in.
Are Designed To Get Your Project Moving Quickly And Efficiently.
Easily fill out pdf blank, edit, and sign them. Save or instantly send your. Intake form for fda/ohrp inspection requests. Web complete fda sop template online with us legal forms.
Food And Drug Administration Office Of Regulatory Affairs.
Any optional section that does not have content will be annotated. Web inspection preparedness standard operating procedure template sop number: Gain the knowledge and tools to assess your existing sops for the most common issues. Web fda sends warning letters to companies for not having adequate sops, as well as not following them.
By Fda, After The Establishment Registration Required In This.
Web download software quality assurance sops and computer system validation templates. Use sops to show compliance with some of the following standards and regulations: Web alvin tai wanted to help companies comply with fda and iso standards, so he started releasing free. Purpose/policy this document describes the medical device single audit program.