Drawing Of Hydrogen Atom
Drawing Of Hydrogen Atom - Therefore, if we make a proton the size of the picture above, 1000 pixels across, then the electron orbiting this proton is located 50,000,000 pixels to the right (but could be found anywhere in the sphere around the. Hydrogen is the most abundant of all elements in the universe. The overall shape of the molecule is a pyramid with nitrogen at the vertex and a triangular base formed by the three hydrogen atoms. The energy levels presented correspond with. Hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms.
Distinguish between the bohr and schrödinger models of the atom The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Web hydrogen atom ground state. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Therefore, if we make a proton the size of the picture above, 1000 pixels across, then the electron orbiting this proton is located 50,000,000 pixels to the right (but could be found anywhere in the sphere around the. Web the hydrogen spectrum is often drawn using wavelengths of light rather than frequencies. 1), the most common isotope of the element hydrogen.
Bohr Atomic Model Of Hydrogen
Distinguish between correct and incorrect features of the bohr model, in light of modern quantum mechanics. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells,.
[DIAGRAM] Labeled Diagram Of Hydrogen Atom
Distinguish between the bohr and schrödinger models of the atom Web the nitrogen atom is depicted as the larger, central blue sphere, and the three hydrogen atoms are depicted as the smaller white spheres off to the sides, which form a kind of tripod. Web hydrogen atom, 1 h; Web bohr's model of hydrogen is.
Describe Bohr’s model of the hydrogen atom. bitWise Academy
Identify the physical significance of each of the quantum numbers (n, l, m) of the hydrogen atom; Distinguish between the bohr and schrödinger models of the atom Web the hydrogen spectrum is often drawn using wavelengths of light rather than frequencies. The overall shape of the molecule is a pyramid with nitrogen at the vertex.
How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
This expression denotes a parabola. Unfortunately, because of the mathematical relationship between the frequency of light and its wavelength, two completely different views of the spectrum are obtained when it is plotted against frequency or against wavelength. Distinguish between the bohr and schrödinger models of the atom Electron configuration the arrangements of electrons above the.
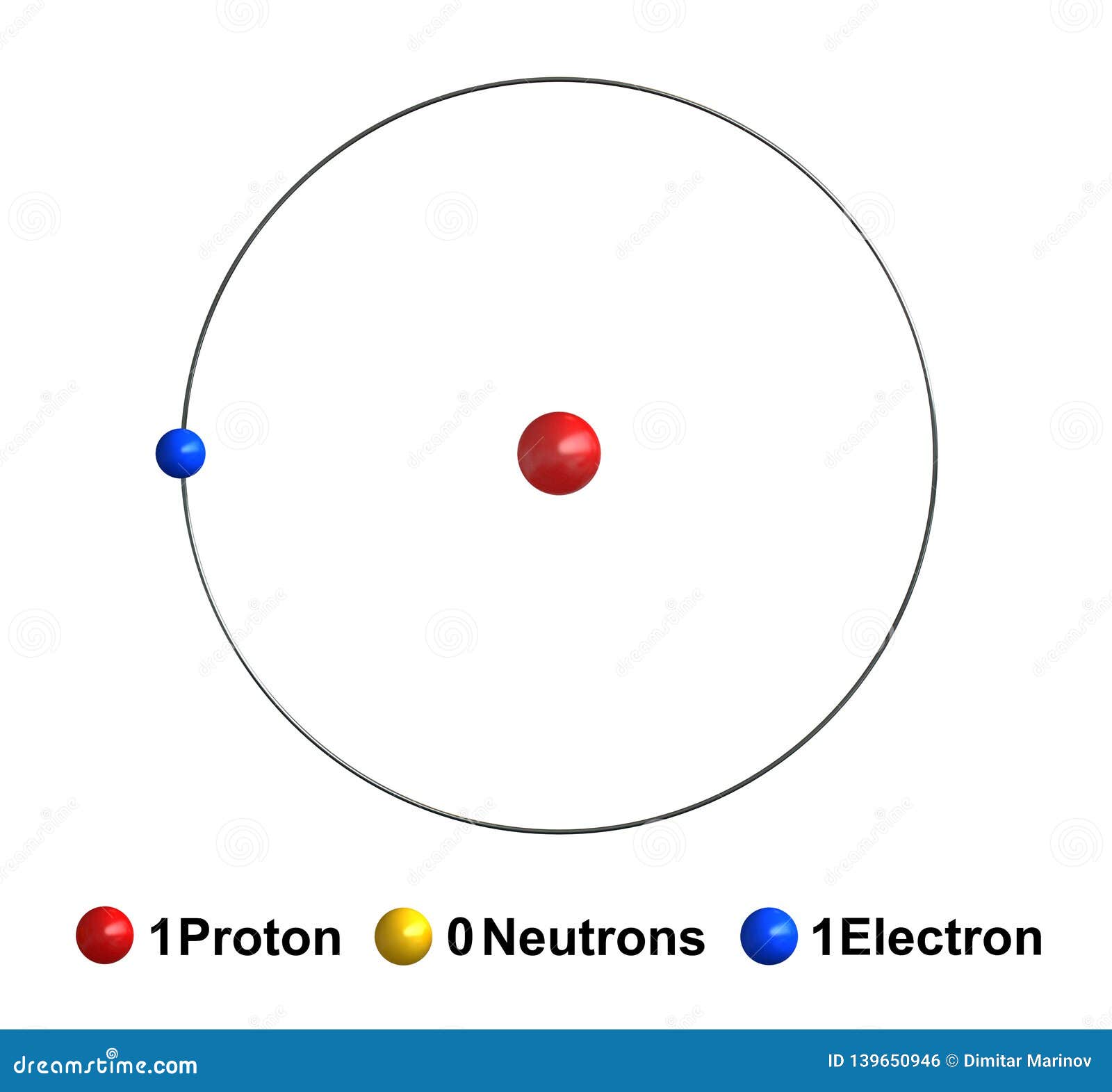
Hydrogen stock illustration. Illustration of education 139650946
Its nucleus consists of a single proton (red) and no neutrons. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Explain bohr’s theory of the hydrogen atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. It's these.
Hydrogen Atom Diagram Concept Stock Vector Illustration of abstract
Hydrogen is the most abundant of all elements in the universe. Web describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum; Explain bohr’s theory of the hydrogen atom. Web the hydrogen spectrum is often drawn using wavelengths of light rather than frequencies. Web hydrogen energy levels. Therefore,.
Diagram representation element hydrogen Royalty Free Vector
Web diagram of a simple hydrogen atom. The energy of a free (nearly free) electron in a solid (say, metals) is given by e =ℏ2k2/2m e = ℏ 2 k 2 / 2 m where k k is the momentum vector, ℏ ℏ is planck's constant and m m is the mass of the electron..
Hydrogen atom on white background Royalty Free Vector Image
The heavier elements were originally made from hydrogen atoms or from other elements that were originally made from hydrogen atoms. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. The image below represents shell structure, where each shell is associated with principal quantum number.
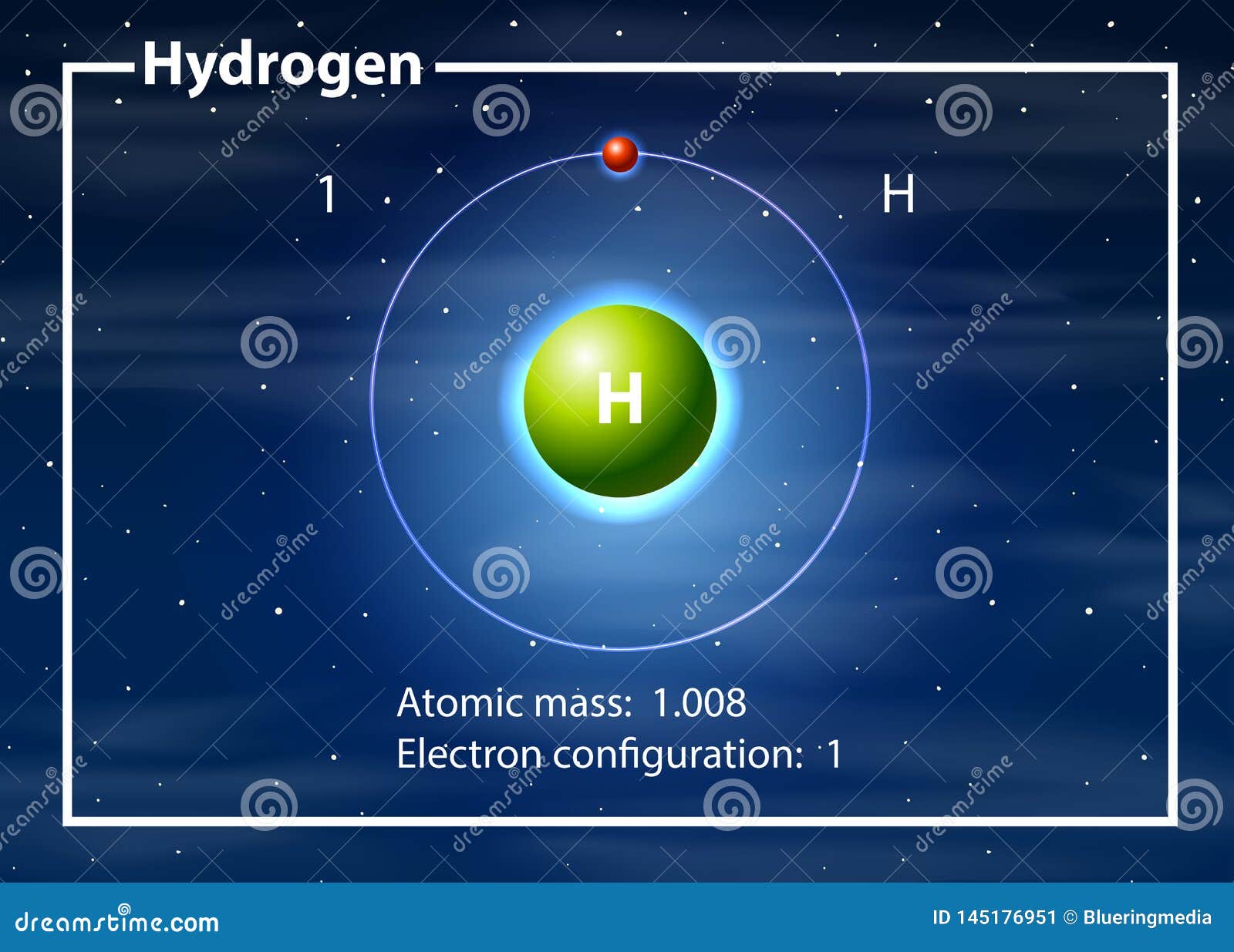
Hydrogen Definition, Structure, Properties & Uses Embibe
Distinguish between the bohr and schrödinger models of the atom The image below represents shell structure, where each shell is associated with principal quantum number n. The energy levels presented correspond with. Unfortunately, because of the mathematical relationship between the frequency of light and its wavelength, two completely different views of the spectrum are obtained.
Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
Hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around.
Drawing Of Hydrogen Atom Web the hydrogen spectrum is often drawn using wavelengths of light rather than frequencies. The energy levels presented correspond with. Web this web page shows the scale of a hydrogen atom. Web hydrogen atom, 1 h; Distinguish between correct and incorrect features of the bohr model, in light of modern quantum mechanics.
Distinguish Between The Bohr And Schrödinger Models Of The Atom
7 288.969 ± 0.001 kev: The overall shape of the molecule is a pyramid with nitrogen at the vertex and a triangular base formed by the three hydrogen atoms. A very simple drawing of an hydrogen. Isotopes of hydrogen complete table of nuclides
The Bohr Model Is Used To Describe The Structure Of Hydrogen Energy Levels.
We will illustrate some of these for the 1s ground state. 5.5k views 8 years ago. It's these hydrogen bonds that give water many of its properties. Its nucleus consists of a single proton (red) and no neutrons.
The Energy Of A Free (Nearly Free) Electron In A Solid (Say, Metals) Is Given By E =ℏ2K2/2M E = ℏ 2 K 2 / 2 M Where K K Is The Momentum Vector, ℏ ℏ Is Planck's Constant And M M Is The Mass Of The Electron.
Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Web describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum; Electron configuration the arrangements of electrons above the last (closed shell) noble gas.
The Image Below Represents Shell Structure, Where Each Shell Is Associated With Principal Quantum Number N.
Web the number of protons in an atom. Web the electron’s speed is largest in the first bohr orbit, for n = 1, which is the orbit closest to the nucleus. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Hydrogen is the most abundant of all elements in the universe.


![[DIAGRAM] Labeled Diagram Of Hydrogen Atom](https://thumbs.dreamstime.com/z/diagram-representation-element-hydrogen-illustration-59013305.jpg)