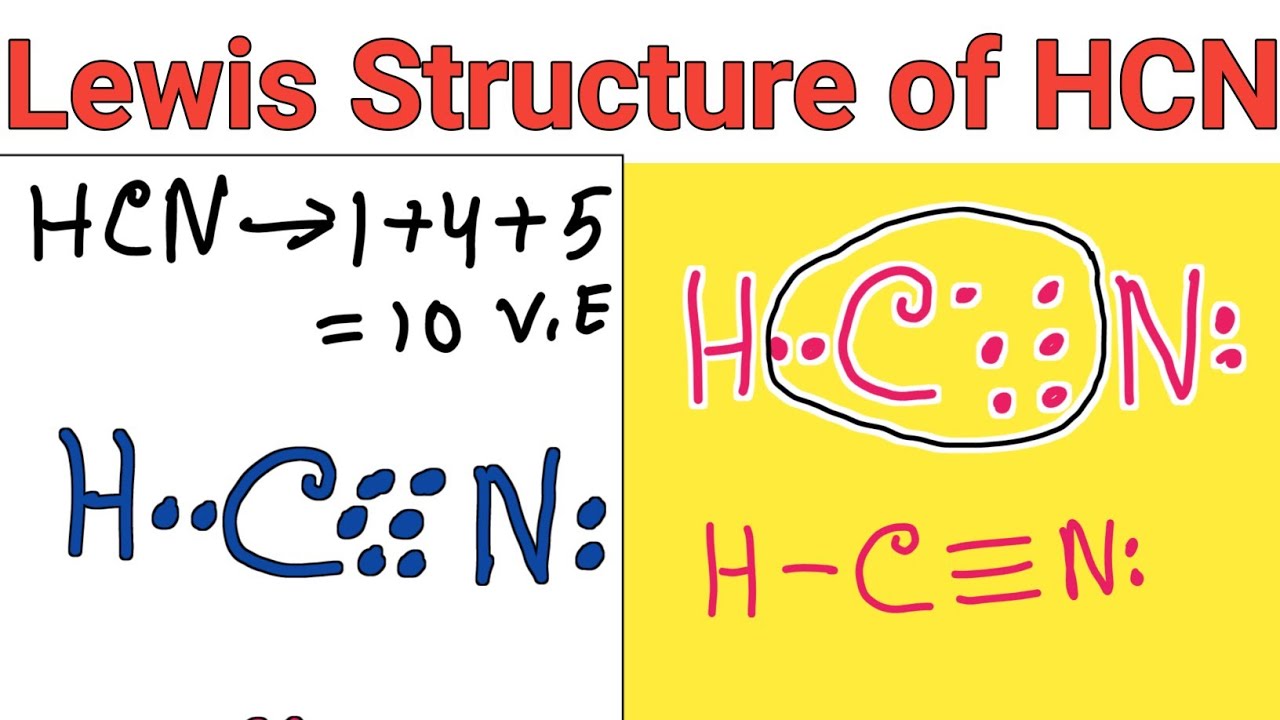
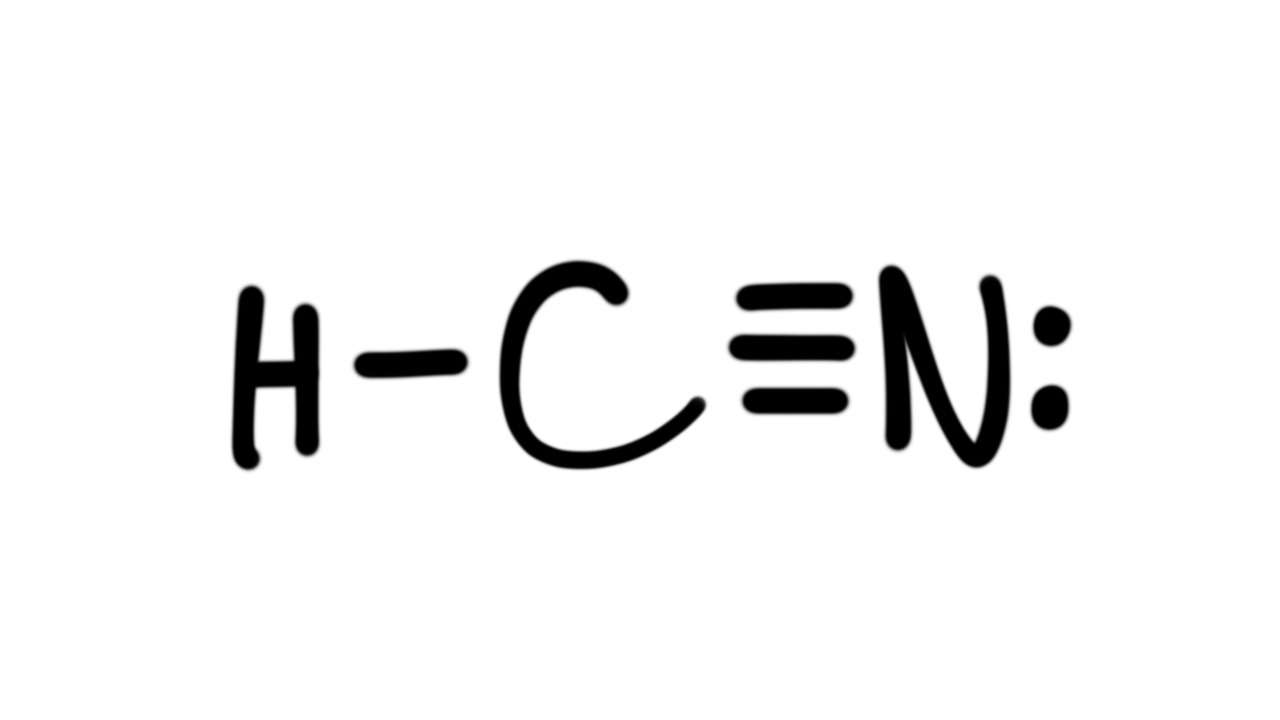Draw The Lewis Structure Of Hcn
Draw The Lewis Structure Of Hcn - Does this molecule exhibit resonance? After determining how many valence electrons there are in hcn, place. The second step is to add valence electrons to the. Draw the skeletal structure showing how the atoms are connected using single bonds. Web science chemistry chemistry questions and answers draw the lewis structure for hcn.
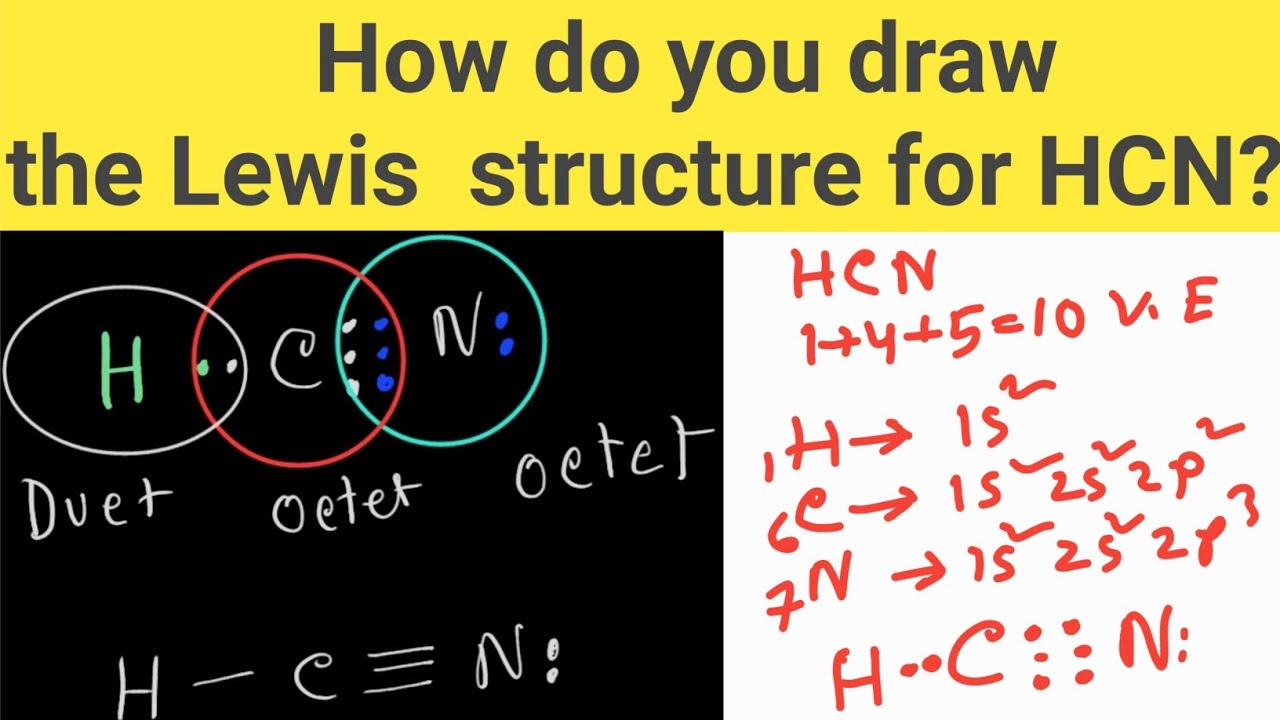
Web science chemistry chemistry questions and answers draw the lewis structure for hcn. Web steps of drawing hcn lewis structure step 1: Web draw the lewis structure of hcn. Web first, let's do hydrogen cyanide, the poison that might have killed lewis. Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen. Web to draw the hcn lewis structure, follow these steps: Web steps #1 first draw a rough sketch.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
The second step is to add valence electrons to the. Put the least electronegative atom c in the middle with h and cl on either side. For the hcn lewis structure we have one valence electron for hydrogen, we have four for carbon, and we have five for nitrogen, for a total of ten valence.
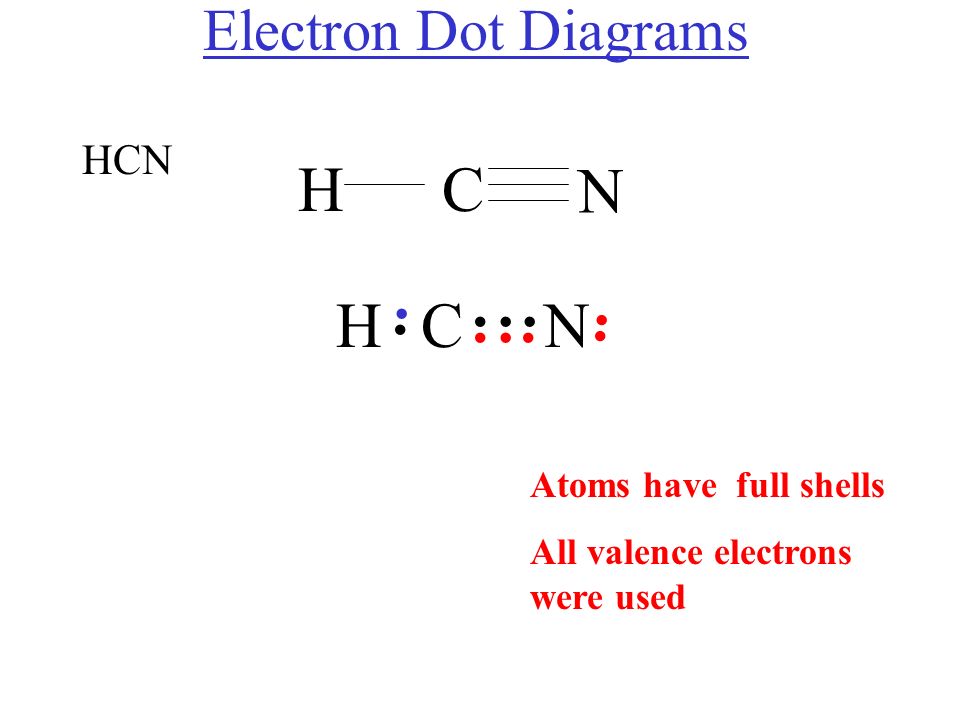
Lewis Diagram For Hcn
#2 mark lone pairs on the atoms. Web the first step is to sketch the lewis structure of the hcn molecule, to add valence electrons around the cyanide species; For the hcn lewis structure we have one valence electron for hydrogen, we have four for carbon, and we have five for nitrogen, for a total.
Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule
Count the valence electrons you. Web draw the lewis structure of hcn. Determine the total number of valence electrons by adding the valence electrons of each atom in the molecule. Find the total valence electrons in hcn molecule. In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen. Usually.
HCN Lewis Structure (Hydrogen Cyanide) YouTube
For the hcn lewis structure we have one valence electron for hydrogen, we have four for carbon, and we have five for nitrogen, for a total of ten valence electrons. Web steps #1 first draw a rough sketch. After determining how many valence electrons there are in hcn, place. Web for the hcn lewis structure,.
Hydrogen Cyanide YouTube
Web draw out a correct lewis structure for the following compounds. In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen. In this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Web steps #1 first draw a rough sketch. Here.
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN
Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web the first step is to sketch the lewis structure of the hcn molecule, to add valence electrons around the cyanide species; #2 mark lone pairs on the atoms. In order to find the total valence electrons in.
Lewis Diagram For Hcn
Web to draw the hcn lewis structure, follow these steps: Web draw a skeleton structure. Web hcn lewis structure. Web the first step is to sketch the lewis structure of the hcn molecule, to add valence electrons around the cyanide species; Does this molecule exhibit resonance? Web steps #1 first draw a rough sketch. In.
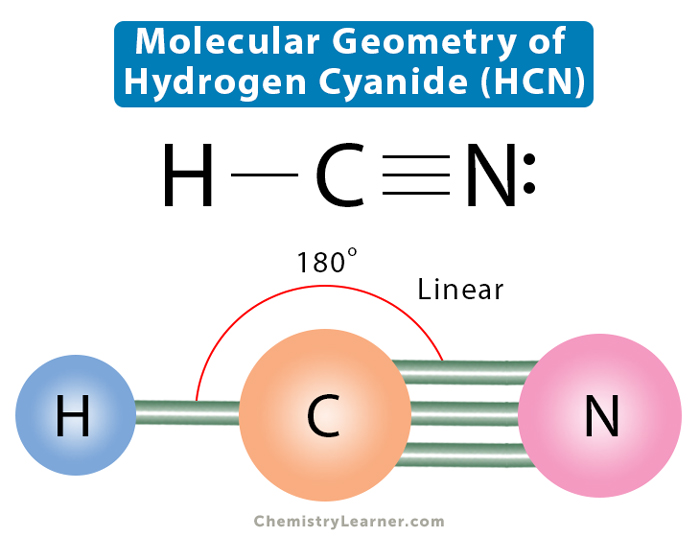
Molecular Geometry, Lewis Structure, and Bond Angle of HCN
This problem has been solved! After determining how many valence electrons there are in hcn, place. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen. Web hcn lewis structure. Lewis structure.
HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
Count the valence electrons you. After determining how many valence electrons there are in hcn, place. Web steps #1 first draw a rough sketch. Put the least electronegative atom c in the middle with h and cl on either side. Web hcn lewis structure. Web the first step is to sketch the lewis structure of.
Lewis structure of HCN (Hydrogen cyanide) YouTube
#2 mark lone pairs on the atoms. Usually try to draw the most symmetrical structure. In order to find the total valence electrons in hcn molecule,. In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen. Here we have to find the valence electrons of all three atoms, hydrogen,.
Draw The Lewis Structure Of Hcn In this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. After determining how many valence electrons there are in hcn, place. Put the least electronegative atom c in the middle with h and cl on either side. Web draw the lewis structure of hcn. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule.
Web Draw The Lewis Dot Structure Of Hydrogen Cyanide (Hcn) Molecule.
Web science chemistry chemistry questions and answers draw the lewis structure for hcn. Multiple bonds, hcn matthew gerner 9.62k subscribers subscribe subscribed 3.8k views 6 years ago chem 101:. Web hcn lewis structure. Lewis structure or lewis dot structure helps to figure out the valance electrons or hybridization of any compound.
Here We Have To Find The Valence Electrons Of All Three Atoms, Hydrogen, Carbon, And Nitrogen.
Web draw the lewis structure of hcn. Usually try to draw the most symmetrical structure. #2 mark lone pairs on the atoms. Web the first step is to sketch the lewis structure of the hcn molecule, to add valence electrons around the cyanide species;
This Problem Has Been Solved!
In order to find the total valence electrons in hcn molecule,. Draw the skeletal structure showing how the atoms are connected using single bonds. Find the total valence electrons in hcn molecule. Put the least electronegative atom c in the middle with h and cl on either side.
In This Video, We Will Look At The Lewis Structure Of Hydrogen Cyanide Having A Chemical Formula Of Hcn.
For the hcn lewis structure we have one valence electron for hydrogen, we have four for carbon, and we have five for nitrogen, for a total of ten valence electrons. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. In the periodic table, hydrogen lies in group 1, carbon lies in group 14, and nitrogen. Web to draw the hcn lewis structure, follow these steps: