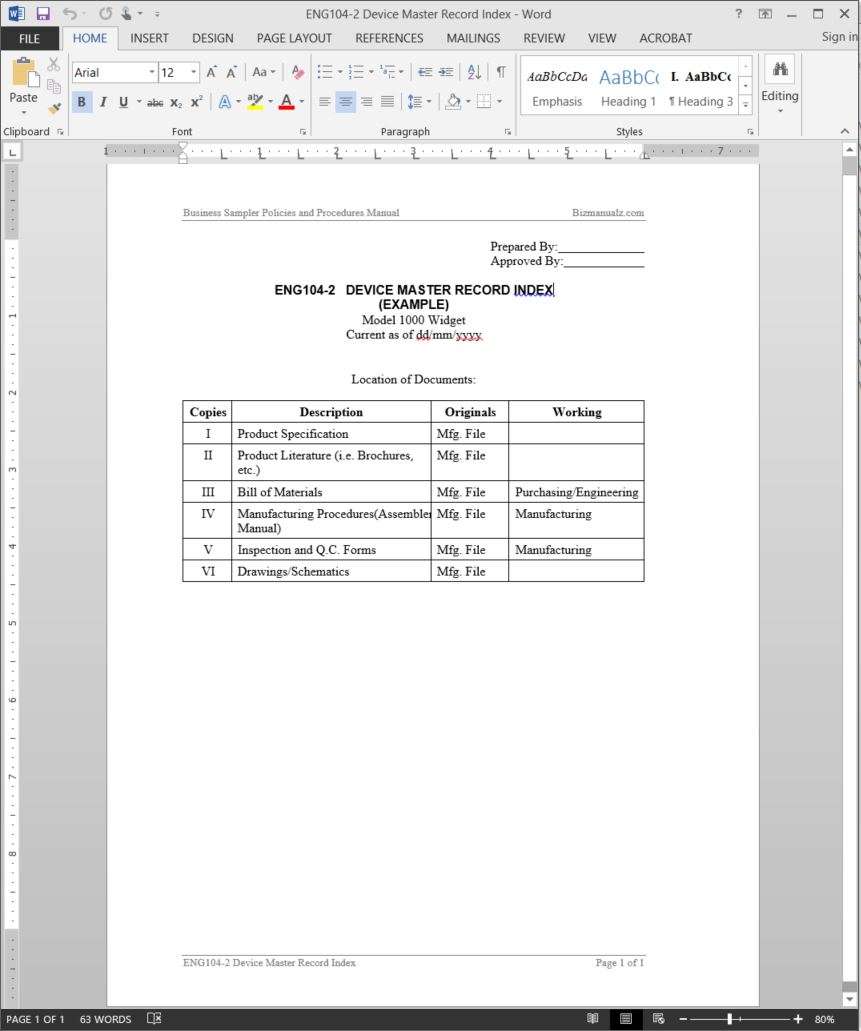
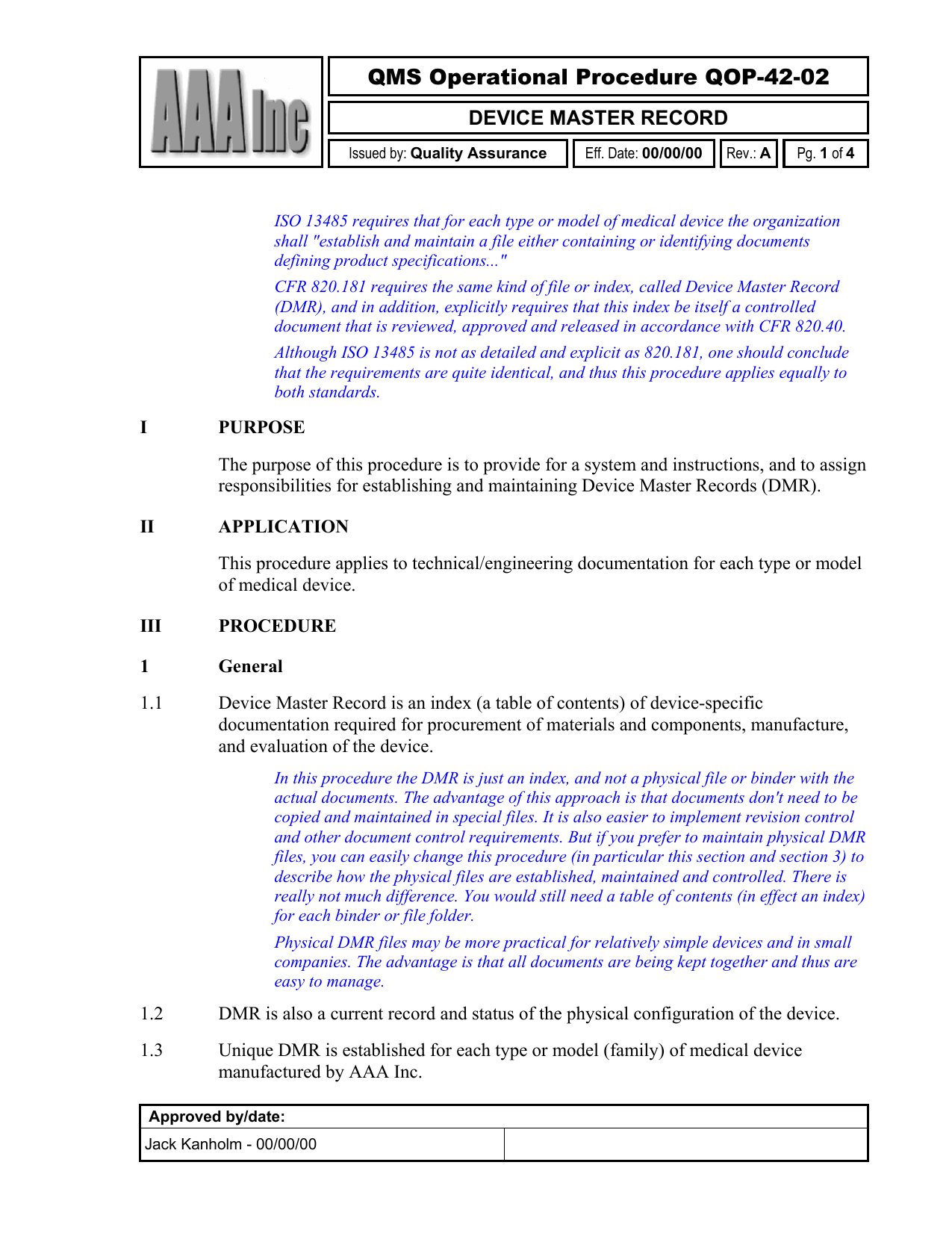
Device Master Record Template
Device Master Record Template - Web a device master record ( dmr) is a compilation of all the instructions, drawings and other records that must be used to. Web #1 can someone share their dmr index structure or format? The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web definitions (21 cfr 820.3) device master record (dmr) compilation of records containing procedures and specifications. Web device master record index template details pages:
The requirement for a device. Design history file, device master record, dhf, dmr description reviews (0) description Web a device expert record (dmr) contains show this information required to create your device from start to finish. Microsoft word 2013 (.docx) language:. Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device. Web no name medical example 1 device master record anycity, georgia 30000 document #: Everything you need to know to build and.
DEVICE MASTER RECORD SOP Template MD21 GMP, QSR & ISO Comp
| medical device validation, regulation,. Everything you need to know to build and. The dhr typically contains, for example, design plan, design review, verification & validation test plan and test reports, design transfer, etc. This package includes one example/template dmr and one dhf. The dmr is basically considered the collection of all the information needed.
Medical Device Master File Template
The dmr is the device master record. Microsoft word 2013 (.docx) language:. Web for a device master record (dmr), i recommend creating a dmr index using a template that is organized in accordance with an international. Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of.
Device Master Record Procedure
Web the device master record is a document requested according to fda 21 cfr 820 regulation and having wall organised device master record example and template is essential. $189.00 emailed in pdf format product code: Design history file, device master record, dhf, dmr description reviews (0) description Web the food and drug administration (fda) requires.
Device Master Records.doc Specification (Technical Standard
| medical device validation, regulation,. Web a device expert record (dmr) contains show this information required to create your device from start to finish. Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device. $189.00 emailed in pdf format product code: Web the.
Device Master Record Index Template
Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine. Gmp procedures, validation sops & templates tags: Web a device master record (dmr) is a collection of records that contains the procedures and specifications for a finished medical. Web a device master record (.
8 DEVICE MASTER RECORDS
The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine. Web device master record (dmr) means a compilation of records containing the procedures and specifications for.
Medical Device Master File Template alat press tutup gelas plastik murah
This package includes one example/template dmr and one dhf. The requirement for a device. Web the device master record contents template is a listing of items that may appear in a device master record. Web device master record index template details pages: Microsoft word 2013 (.docx) language:. Web the food and drug administration (fda) requires.
Device Master Records & Design History Files
Web device master record (dmr) means a compilation of records containing the procedures and specifications for a finished device. Web definitions (21 cfr 820.3) device master record (dmr) compilation of records containing procedures and specifications. Web a device master record ( dmr) is a compilation of all the instructions, drawings and other records that must.
Device Master Record
Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device. Web definitions (21 cfr 820.3) device master record (dmr) compilation of records containing procedures and specifications. Our contract manufacturer is asking for a dmr index. The dmr is basically considered the collection of.
Device Master Record Contents Template
Web a device master record (dmr) is a collection of records that contains the procedures and specifications for a finished medical. Web device master record (dmr) means a compilation of records containing the procedures and specifications for a finished device. Web #1 can someone share their dmr index structure or format? Web a device master.
Device Master Record Template Web the device master record contents template is a listing of items that may appear in a device master record. Web device master record index template details pages: Web the dhr is the set of documents that demonstrates that the design process of a medical device has been performed according to the design plan and as per applicable regulations. The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web the requirements and solutions, which are adopted during a review for device improvement, are documented in the.
Everything You Need To Know To Build And.
Web $ 29.95 this procedure describes the requirements for device master records (dmrs) and design history files (dhfs). Gmp procedures, validation sops & templates tags: This package includes one example/template dmr and one dhf. Design history file, device master record, dhf, dmr description reviews (0) description
Web The Dhr Is The Set Of Documents That Demonstrates That The Design Process Of A Medical Device Has Been Performed According To The Design Plan And As Per Applicable Regulations.
$189.00 emailed in pdf format product code: Web the food and drug administration (fda) requires manufacturers of medical devices to create and maintain. | medical device validation, regulation,. Web a device master record (dmr) contains all the information required to build your device from start to close.
Web For A Device Master Record (Dmr), I Recommend Creating A Dmr Index Using A Template That Is Organized In Accordance With An International.
Web device master record index template details pages: The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web device master record (dmr) means a compilation of records containing the procedures and specifications for a finished device. The dmr is the device master record.
Web The Device Master Record Is A Document Requested According To Fda 21 Cfr 820 Regulation And Having Wall Organised Device Master Record Example And Template Is Essential.
Web #1 can someone share their dmr index structure or format? Web a device expert record (dmr) contains show this information required to create your device from start to finish. Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine. Our contract manufacturer is asking for a dmr index.