Clinical Trial Protocol Synopsis Template
Clinical Trial Protocol Synopsis Template - Web the clinical trial template has site lists of libraries for clinical trial protocols, protocol documents,. The interventional drug/device trial template and the. Web intervention study template (clinical trials): Web download protocol synopsis template (dutch) patient facing documents can also be uploaded in this section of ctis. Web nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank.
This template is intended to be used for clinical trials. The interventional drug/device trial template and the. Nih applicants can use a template with instructional and sample text to help. Web efficiently develop a clear and concise plain language protocol synopsis for every clinical trial, to help all stakeholders quickly. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the. Web there are two templates to be used for interventional research: Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the “recipe” for a successful clinical trial that.
Clinical Trial Protocol Template Australia Templates NjQyMDk
Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the. The schedule of events is a tabular description. Web they include the following; Web efficiently develop a clear and concise plain language protocol synopsis for every clinical trial, to help all stakeholders quickly. Web download protocol synopsis template.
Clinical Trial Protocol Summary Template
This template is intended to be used for clinical trials. Web intervention study template (clinical trials): The interventional drug/device trial template and the. Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the “recipe” for a successful clinical trial that. Web nci informed consent template for ctep.
Clinical Trial Protocol Synopsis Template
Web the clinical trial template has site lists of libraries for clinical trial protocols, protocol documents,. This template is intended to be used for clinical trials. In order to have a clear roadmap, it is important for the investigator to have a. Web there are two templates to be used for interventional research: Web protocol.
Clinical Trial Protocol Format
Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web download protocol synopsis template (dutch) patient facing documents can also be uploaded in this section of ctis. Cirm clinical protocol synopsis template study title provide full title of the study clinical phase. Web.
Protocol Synopsis Sample Sheet (HW) PDF C Reactive Protein
Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the. Web nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank. Cirm clinical protocol synopsis template study title provide full title of the study clinical phase. Web sample protocol.
Phylotocol template. Based on the NIH clinical trial protocol, the
Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the. Web protocol templates for clinical trials. Web there are two templates to be used for interventional research: Nih applicants can use a template with instructional and sample text to help. Web download protocol synopsis template (dutch) patient facing.
Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
The interventional drug/device trial template and the. This template is intended to be used for clinical trials. Web a clinical research protocol is a roadmap. The goal of the study, the details on tests, treatments, and procedures, the eligibility. In order to have a clear roadmap, it is important for the investigator to have a..
Template For Trial Exhibit How to Create a Clinical Trial Recruitment
Web efficiently develop a clear and concise plain language protocol synopsis for every clinical trial, to help all stakeholders quickly. Web protocol templates for clinical trials. Web sample protocol templates and resources: Web they include the following; Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the.
Clinical Study Protocol (CSP) Template Clinical Study Templates
Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the “recipe” for a successful clinical trial that. Web the clinical trial template has site lists of libraries for clinical trial protocols, protocol documents,. Web protocol templates for clinical trials. Web protocol version 8.0 dated 18 mar 2020.
(PDF) Development and Implementation of Clinical Trial Protocol
Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the “recipe” for a successful clinical trial that. Web protocol templates for clinical trials. Web nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank. The interventional drug/device trial.
Clinical Trial Protocol Synopsis Template The schedule of events is a tabular description. This template is intended to be used for clinical trials. Web protocol version 8.0 dated 18 mar 2020 page 1 of 90 protocol template_version 1.2 clinical trial protocol open label. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the. Web protocol templates for clinical trials.
Web Download Protocol Synopsis Template (Dutch) Patient Facing Documents Can Also Be Uploaded In This Section Of Ctis.
Web nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank. The interventional drug/device trial template and the. Web the synopsis is your tool, your map to writing an excellent protocol, and the protocol is the “recipe” for a successful clinical trial that. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national.
Web Efficiently Develop A Clear And Concise Plain Language Protocol Synopsis For Every Clinical Trial, To Help All Stakeholders Quickly.
Web nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank. Web they include the following; Web the protocol synopsis is an overview/summary of the protocol. Nih applicants can use a template with instructional and sample text to help.
Web There Are Two Templates To Be Used For Interventional Research:
Web this clinical trial agreement template makes that process easier by streamlining the process of creating a contract between a. Web protocol version 8.0 dated 18 mar 2020 page 1 of 90 protocol template_version 1.2 clinical trial protocol open label. In order to have a clear roadmap, it is important for the investigator to have a. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the.
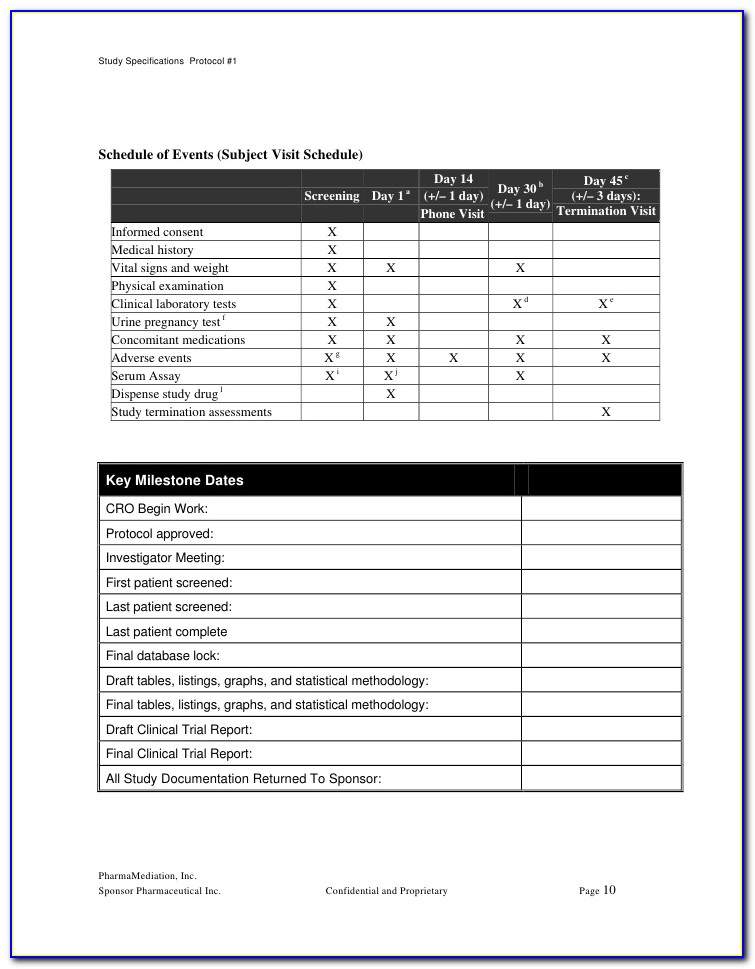
The Schedule Of Events Is A Tabular Description.
This template is intended to be used for clinical trials. Web intervention study template (clinical trials): Cirm clinical protocol synopsis template study title provide full title of the study clinical phase. Web sample protocol templates and resources: