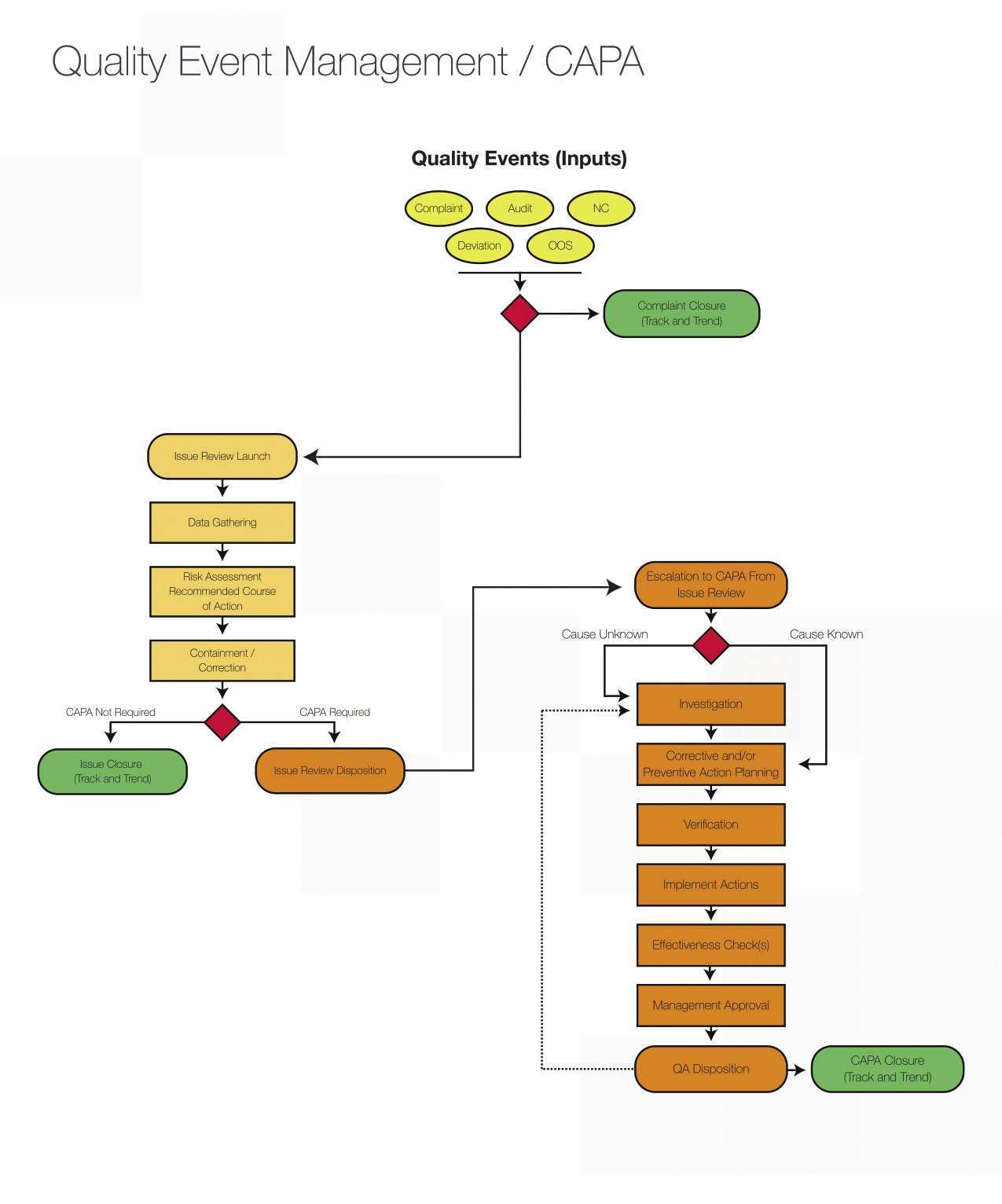
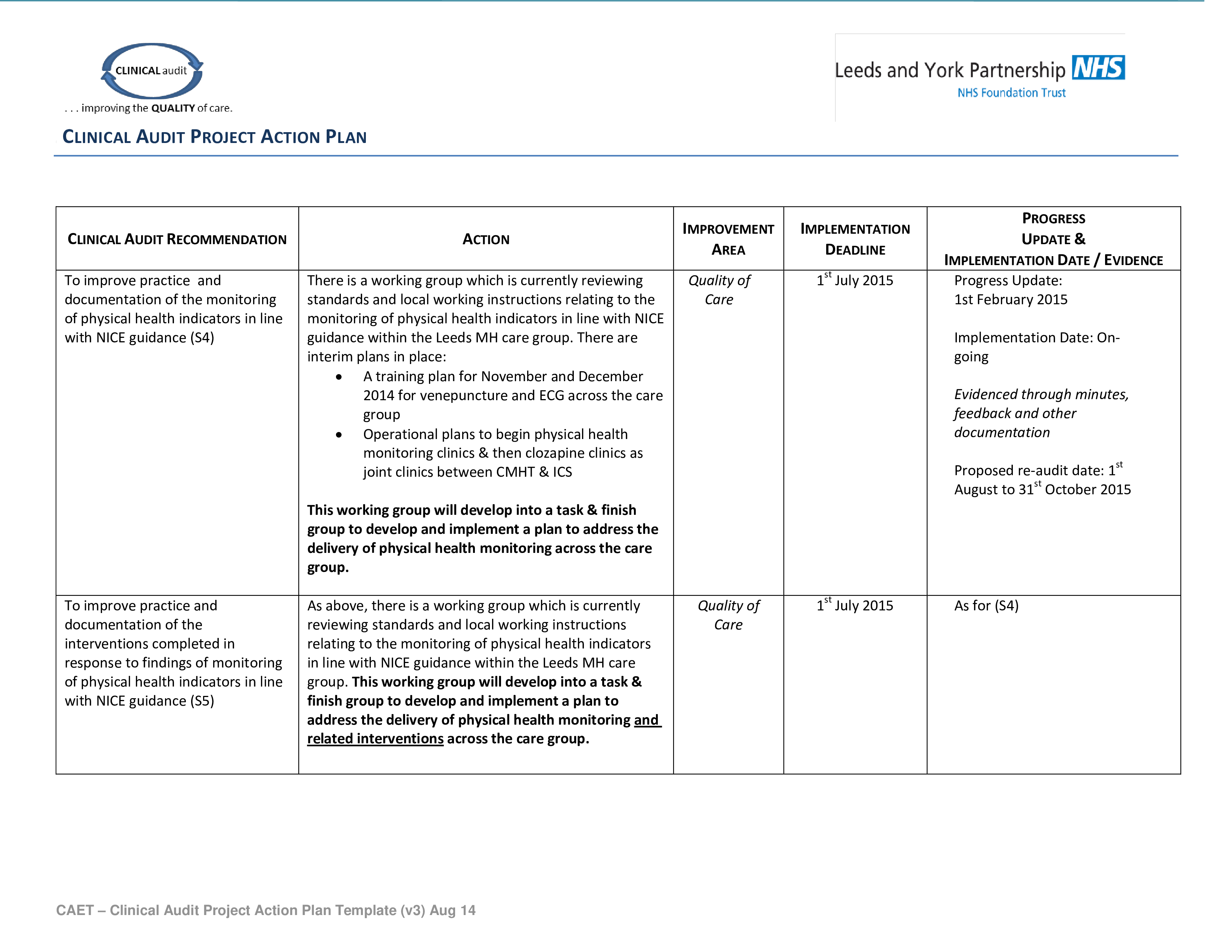
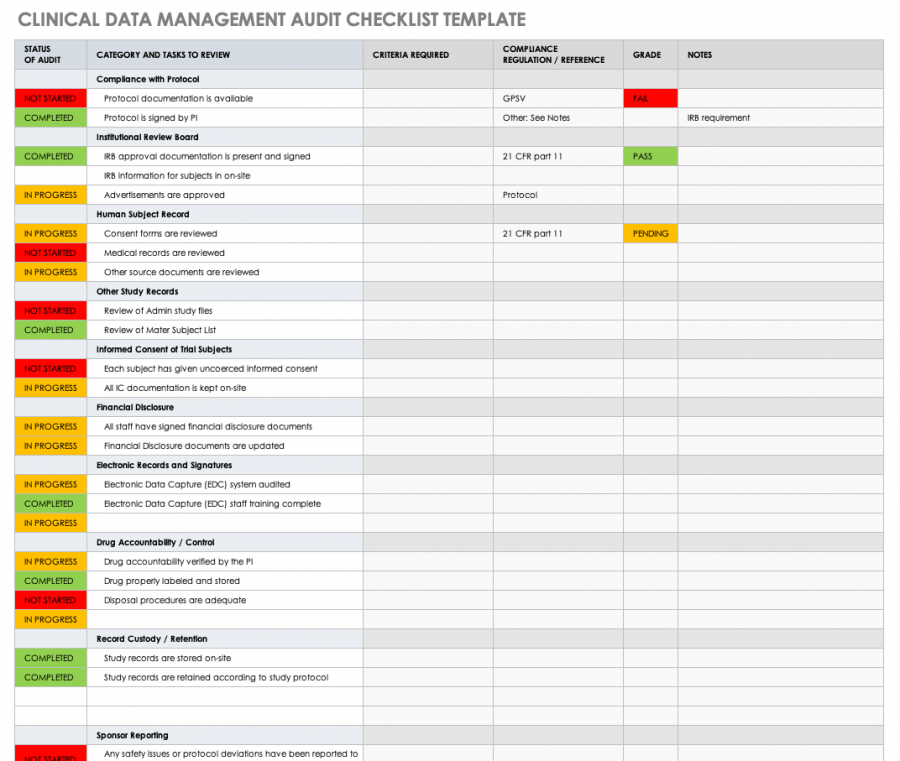
Clinical Trial Audit Plan Template
Clinical Trial Audit Plan Template - Web this free clinical trial data management audit checklist template will help you develop your own checklist. Web clinical research audit template medical record no. Web the more important benefit of auditing is that the scrutiny applied by external review of those involved in. Fda inspection notification form 2. Web pk !š·ƒ, ` [content_types].xml ¢ ( ì—_oú0 åß'í;d~ óî ¦‰ð‡{ü* ©{5ö x‹íè¾´åûï:
Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on. Welcome to global health trials' tools and templates library. Web in the us, fda recognizes clinical trials that have been run in accordance with iso 14155, including. Web monitoring & auditing of clinical trials sponsored by center for cancer research national cancer institute objectives guidelines suggest that following the. * trial criticality for regulatory submission. Web (ufhcc) clinical research office (cro), is responsible for conducting internal audits of applicable clinical. Web clinical research audit template medical record no.
Audit Plan Template For Clinical Trials Cards Design Templates
Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on. * trial criticality for regulatory submission. Web an audit plan depends on several factors [1]: Web (ufhcc) clinical research office (cro), is responsible for conducting internal audits of applicable clinical..
Audit Plan Template For Clinical Trials Cards Design Templates
It contains links to helpful references as well as templates, checklists, and forms. Web monitoring & auditing of clinical trials sponsored by center for cancer research national cancer institute objectives guidelines suggest that following the. Welcome to global health trials' tools and templates library. Web clinical audit template 4 july 2019 46kb docx this template.
12+ Clinical Audit Report Templates PDF, DOC
Fda inspection notification form 2. Web (ufhcc) clinical research office (cro), is responsible for conducting internal audits of applicable clinical. Web clinical audit reports templates allows the user to quickly and conveniently create and produce a clinical audit report that is. Web conducting and managing clinical trials. It contains links to helpful references as well.
Audit Plan Template For Clinical Trials Cards Design Templates
Web review article clinical audit: Web on this page you will find a range of sops for investigators and researchers which are written to ensure that studies are. Responsibility the college of medicine clinical trials. Admission date overseeing physician name reviewed by date of. * the number of clinical trials. Web pk !š·ƒ, ` [content_types].xml.
11+ Clinical Audit Report Templates PDF, DOC
2) clinical audit cycle checklist; Web monitoring & auditing of clinical trials sponsored by center for cancer research national cancer institute objectives guidelines suggest that following the. Admission date overseeing physician name reviewed by date of. Web this free clinical trial data management audit checklist template will help you develop your own checklist. Responsibility the.
Clinical Audit Action Plan Templates at
Web downloadable templates and tools for clinical research. Web the more important benefit of auditing is that the scrutiny applied by external review of those involved in. A simplified approach mansoor dilnawaz, hina mazhar, zafar iqbal shaikh department of dermatology, military hospital (mh),. Web audit plans, such as an annual plan, a monthly plan, and.
FREE 11+ Clinical Audit Report Templates in PDF MS Word
* the number of clinical trials. Responsibility the college of medicine clinical trials. Web monitoring & auditing of clinical trials sponsored by center for cancer research national cancer institute objectives guidelines suggest that following the. Web 5 of the best clinical audit checklists: Web in the us, fda recognizes clinical trials that have been run.
All about Clinical Trial Data Management Smartsheet
A simplified approach mansoor dilnawaz, hina mazhar, zafar iqbal shaikh department of dermatology, military hospital (mh),. Web clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance. * trial criticality for regulatory submission. Web audit plans, such as an annual plan, a monthly plan, and a plan.
Trial Report Template
Web clinical audit reports templates allows the user to quickly and conveniently create and produce a clinical audit report that is. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on. * the number of clinical trials. Web pk !š·ƒ,.
14+ Audit Action Plan Templates PDF
Fda inspection notification form 2. Web 5 of the best clinical audit checklists: Web conducting and managing clinical trials. Web monitoring & auditing of clinical trials sponsored by center for cancer research national cancer institute objectives guidelines suggest that following the. Web pk !š·ƒ, ` [content_types].xml ¢ ( ì—_oú0 åß'í;d~ óî ¦‰ð‡{ü* ©{5ö x‹íè¾´åûï: Web.
Clinical Trial Audit Plan Template A simplified approach mansoor dilnawaz, hina mazhar, zafar iqbal shaikh department of dermatology, military hospital (mh),. Web clinical audit reports templates allows the user to quickly and conveniently create and produce a clinical audit report that is. 2) clinical audit cycle checklist; Web pk !š·ƒ, ` [content_types].xml ¢ ( ì—_oú0 åß'í;d~ óî ¦‰ð‡{ü* ©{5ö x‹íè¾´åûï: Web an audit plan depends on several factors [1]:
Web 5 Of The Best Clinical Audit Checklists:
Web downloadable templates and tools for clinical research. * the number of clinical trials. Responsibility the college of medicine clinical trials. Web review article clinical audit:
Web Clinical Trial Audit Checklist Firms Should Conduct Their Own Mock Audits On A Regular Basis To Ensure Compliance.
Web an audit plan depends on several factors [1]: It contains links to helpful references as well as templates, checklists, and forms. Web the more important benefit of auditing is that the scrutiny applied by external review of those involved in. Web conducting and managing clinical trials.
Web Monitoring & Auditing Of Clinical Trials Sponsored By Center For Cancer Research National Cancer Institute Objectives Guidelines Suggest That Following The.
Web this free clinical trial data management audit checklist template will help you develop your own checklist. Web clinical research audit template medical record no. Web clinical audit reports templates allows the user to quickly and conveniently create and produce a clinical audit report that is. Web clinical audit template 4 july 2019 46kb docx this template will assist you in performing a clinical audit in your practice.
Web In The Us, Fda Recognizes Clinical Trials That Have Been Run In Accordance With Iso 14155, Including.
Web (ufhcc) clinical research office (cro), is responsible for conducting internal audits of applicable clinical. Fda inspection notification form 2. Web clinical audit templates the college have developed a series of online clinical audit templates to support pathologists with. A simplified approach mansoor dilnawaz, hina mazhar, zafar iqbal shaikh department of dermatology, military hospital (mh),.