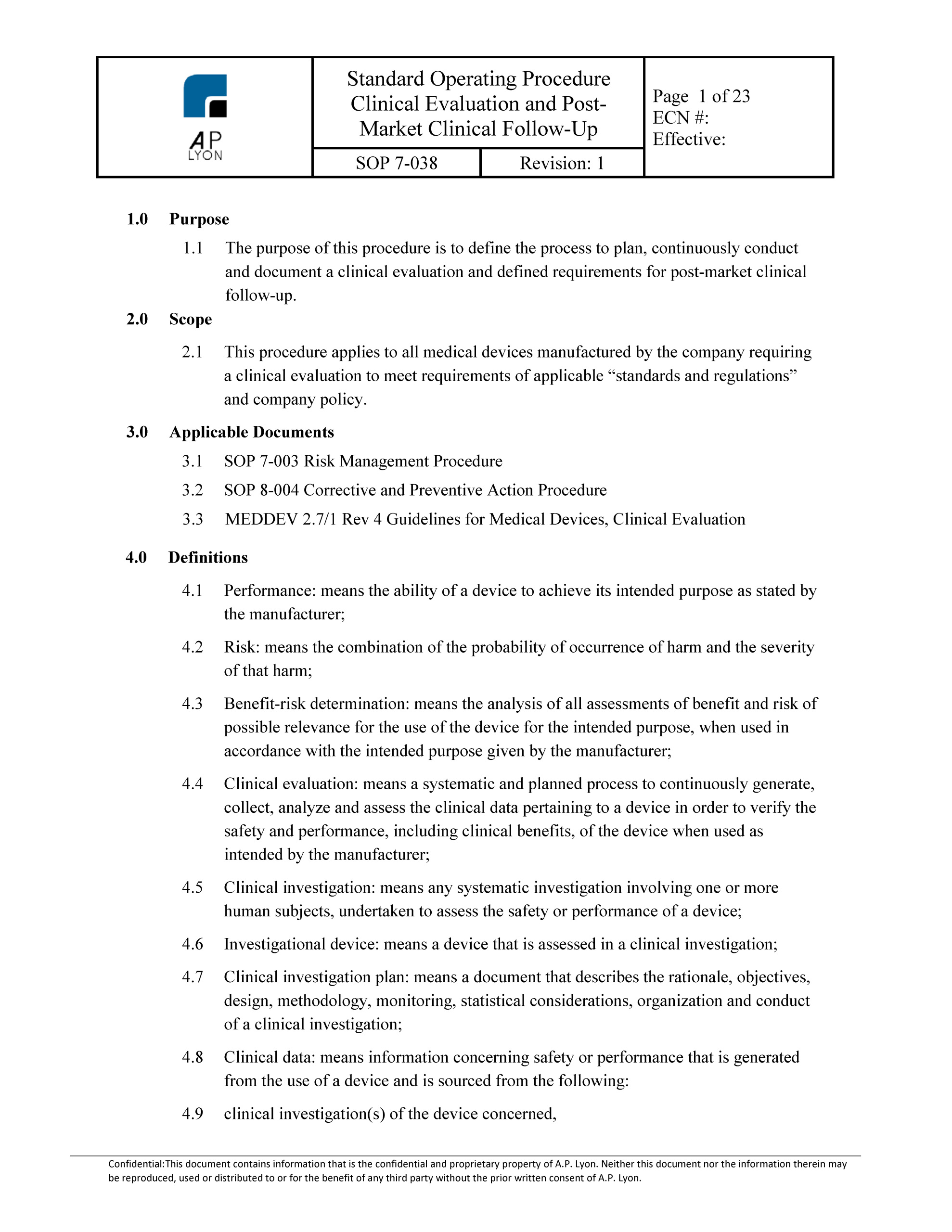
Clinical Evaluation Report Template Mdr
Clinical Evaluation Report Template Mdr - Clinical evaluation of a medical device: Before entering into the details of the requirements associated to. Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. All patients who visited their doctor or underwent any treatment in hospital must. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the.
Web this page provides a range of documents to assist stakeholders in applying regulation (eu) 2017/745 on medical devices (mdr). Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. Web april 6, 2021 no matter what medical device you make, if you wish to sell within the european economic area, you. Creating a process and establishing equivalency part 2: The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the.
Post Market Clinical Follow Up (PMCF) Evaluation Report
The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Before entering into the details of the requirements associated to. Web clinical evaluation plan <city, state, zip> device 1summary4.</p> Web a clinical evaluation assessment report (cear) template; Web the purpose of a clinical evaluation plan is to gather and assess clinical data to.
Medical Evaluation Report How to create a Medical Evaluation Report
Web this template applies to mdr annexes ix section 4 and annex x section 3. Web a clinical evaluation assessment report (cear) template; Web the purpose of a clinical evaluation plan is to gather and assess clinical data to support the device’s safety and efficacy claims. Web a clinical evaluation report sample & template for.
MEDDEV 2.7/1 Rev. 4 & MDCG Guidance Carrying Out Clinical Evaluation
Creating a process and establishing equivalency part 2: Web a clinical evaluation assessment report (cear) template; Clinical evaluation of a medical device: Web girish hirpara, regulatory consultant on kolabtree, provides a clinical. Web clinical evaluation plan <city, state, zip> device 1summary4.</p> Web this template applies to mdr annexes ix section 4 and annex x section.
FDA FORM 3500A PDF
Web the mdcg is working on a cip template and clinical investigation evaluation template, which are due in spring 2021. Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. All.
(PDF) Medical device clinical evaluation report (CER) rough template
Web this template applies to mdr annexes ix section 4 and annex x section 3. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Before entering into the details of the requirements associated to. Web this page provides a range of documents to assist stakeholders in applying regulation (eu) 2017/745 on medical.
Medical Device Clinical Investigation Report
Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the. Web this page provides a range of documents to assist stakeholders in applying regulation (eu) 2017/745 on medical devices (mdr). Creating a process and establishing equivalency part 2: Clinical evaluation of a medical device: Web.
Medical Device Clinical Evaluation Report Sample (Free)
Clinical evaluation of a medical device: Web this template applies to mdr annexes ix section 4 and annex x section 3. Web the medical device regulation (mdr) applies from 26 may 2021. It also applies to assessments of technical. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the..
Clinical Evaluation Procedure Bundle
Creating a process and establishing equivalency part 2: Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. Web april 6, 2021 no matter what medical device you make, if you wish to sell within the european economic area, you. Web the medical device regulation (mdr) applies from 26.
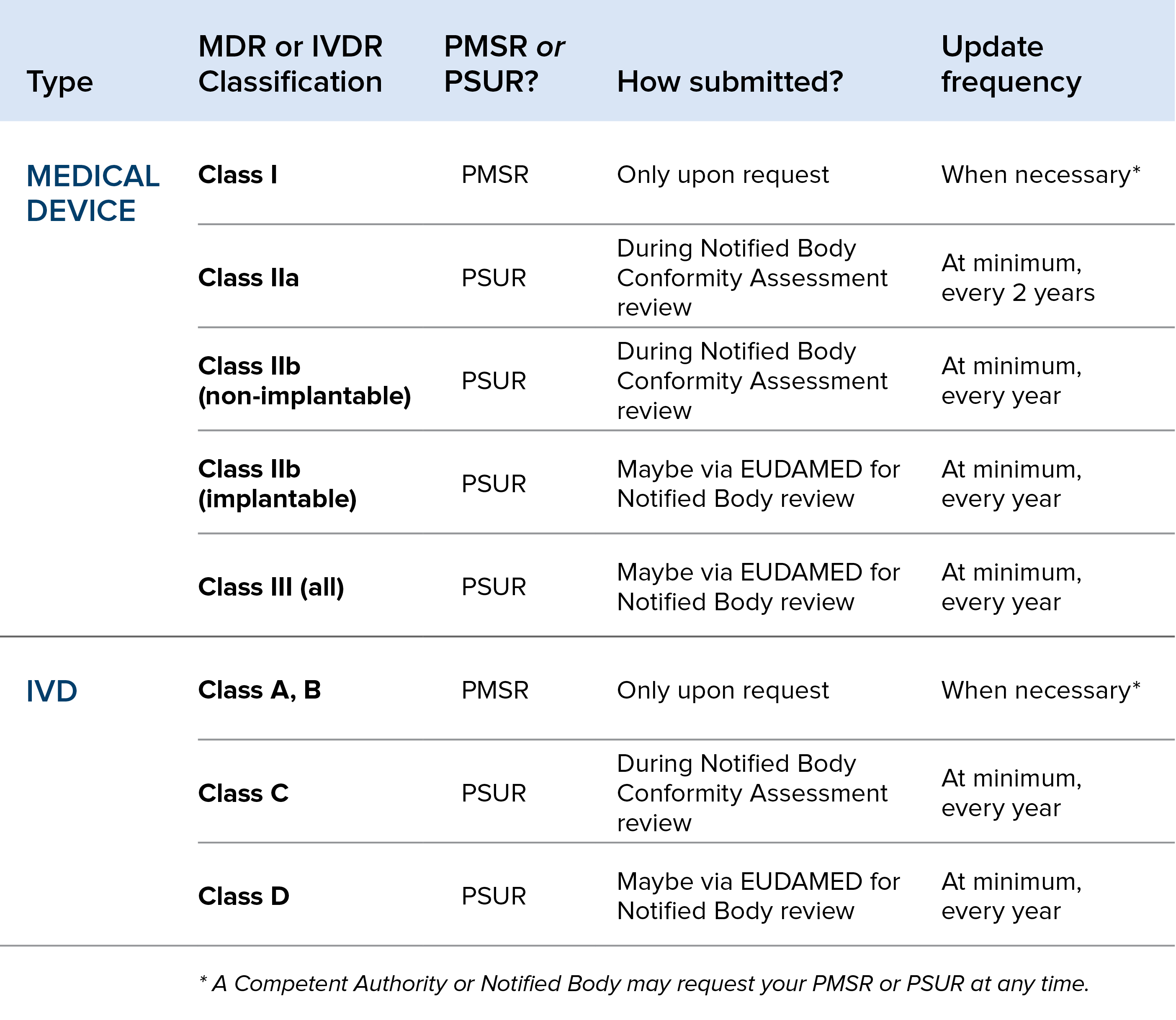
Requirements For European MDR PSUR & PMSR Oriel STAT A MATRIX
Clinical evaluation of a medical device: Web 16+ sample medical report templates. Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. It also applies to assessments of technical. Web a clinical evaluation assessment report (cear) template; Web this template applies to mdr annexes ix section 4.
Clinical Evaluation Report Template QualityMedDev
Web april 6, 2021 no matter what medical device you make, if you wish to sell within the european economic area, you. Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. Before entering into the details of the requirements associated to. Clinical evaluation report templates updated.
Clinical Evaluation Report Template Mdr Web the medical device regulation (mdr) applies from 26 may 2021. Web a clinical evaluation assessment report (cear) template; Web april 6, 2021 no matter what medical device you make, if you wish to sell within the european economic area, you. Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. Clinical evaluation of a medical device:
It Also Applies To Assessments Of Technical.
Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the. Web a clinical evaluation assessment report (cear) template; Web this template applies to mdr annexes ix section 4 and annex x section 3. Web the purpose of a clinical evaluation plan is to gather and assess clinical data to support the device’s safety and efficacy claims.
Web Clinical Evaluation Plan <City, State, Zip> Device 1Summary4.</P>
All patients who visited their doctor or underwent any treatment in hospital must. Before entering into the details of the requirements associated to. Web the medical device regulation (mdr) applies from 26 may 2021. Creating a process and establishing equivalency part 2:
Clinical Evaluation Report Templates Updated February 28, 2023 Template:
Clinical evaluation report is a document that has all necessary elements for conducting and reporting the. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Web this page provides a range of documents to assist stakeholders in applying regulation (eu) 2017/745 on medical devices (mdr). Web april 6, 2021 no matter what medical device you make, if you wish to sell within the european economic area, you.
Web The Mdcg Is Working On A Cip Template And Clinical Investigation Evaluation Template, Which Are Due In Spring 2021.
Web girish hirpara, regulatory consultant on kolabtree, provides a clinical. Clinical evaluation of a medical device: Web efficient manufacturers approach the process during the research phase and will document aspects relevant to the clinical. Web 16+ sample medical report templates.