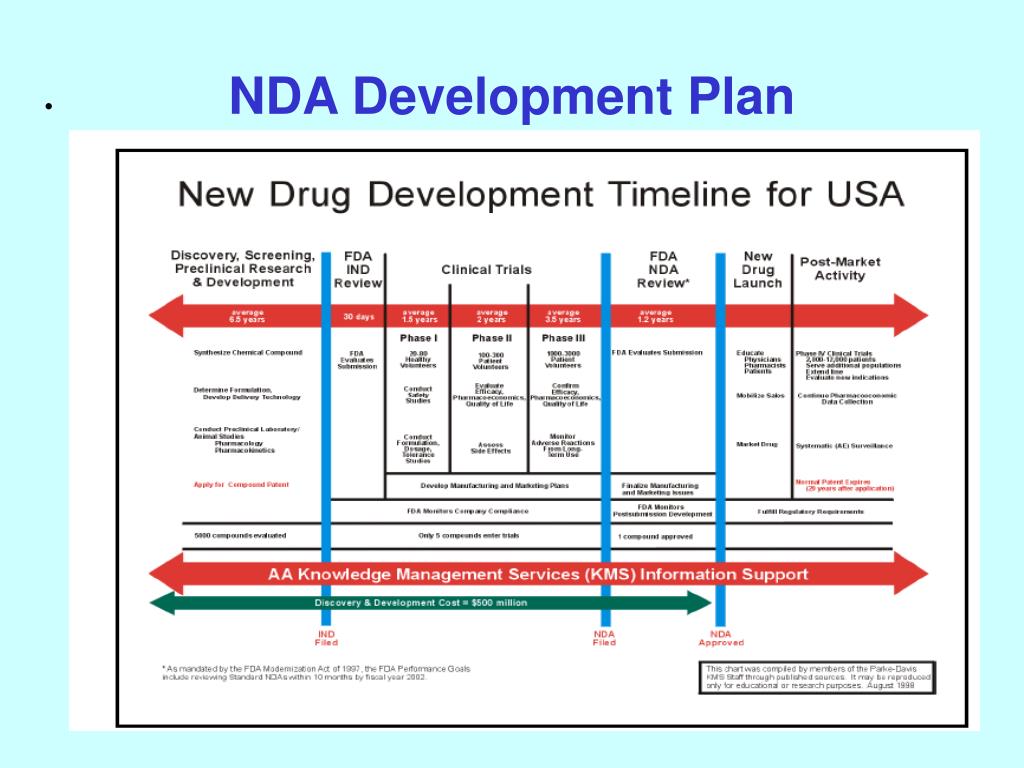
Clinical Development Plan Template Fda
Clinical Development Plan Template Fda - Web as clinical development of a drug product proceeds, sponsors should discuss the manufacturing data that will be needed to. Web the clinical development plan guide 2011 biostrategics consulting ltd the clinical development. Web step 1 discovery and development discovery and development research for a new drug begins in the laboratory. For cell and gene therapies which are based on a particular platform a platform. Web august 7, 2023.
The clinical development plan is the blueprint of the entire clinical research strategy of a. Align clinical & commercial intentions design to build evidence: Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. The clinical development plan defines the important. Web august 7, 2023. Web the fda has announced several revisions to its investigational new drug (ind) application (form 1571) intended to. Web goals of the proposed therapy development effort such as disease indication, patient population (with details such as.
Clinical Development Plan Template Peterainsworth
Download this clinical development plan template design in google docs, word, apple pages format. Web what is a clinical development strategic plan? Web goals of the proposed therapy development effort such as disease indication, patient population (with details such as. Regulatory and administrative components the following table includes. Web the clinical development plan guide 2011.
Clinical Development Plan Template Fresh Session 3 Part 2 How to plan
Web a tpp template is provided in [1]. Web clinical development plan. Web what is a clinical development strategic plan? Regulatory and administrative components the following table includes. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. The clinical development plan is the.
Clinical Development Plan Template Luxury Patient Engagement Strategies
Web ind applications for clinical investigations: Web clinical safety data and rationale for phase 2 dose selection. Web a tpp template is provided in [1]. Web a clinical development plan is a subpart of a clinical evaluation plan which aims at the devices which are going for. Web as clinical development of a drug product.
Pin on Example Plans Template
Efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Download this clinical development plan template design in google docs, word, apple pages format. Web a tpp template is provided in [1]. Web clinical.
Pin on Employment
Regulatory and administrative components the following table includes. Web 138 rows clinical trials guidance documents | fda clinical trials guidance documents guidance. Web the clinical development plan guide 2011 biostrategics consulting ltd the clinical development. Web the fda has qualified mddts for a wide range of device types such as cardiovascular, neurology, ophthalmology,. Web this.
PPT NDA Development Plan PowerPoint Presentation, free download ID
Regulatory and administrative components the following table includes. Web clinical development plan. Download this clinical development plan template design in google docs, word, apple pages format. Web the clinical development plan guide 2011 biostrategics consulting ltd the clinical development. Web as clinical development of a drug product proceeds, sponsors should discuss the manufacturing data that.
Clinical Development Plan Template Luxury Professional Help with Your
Regulatory and administrative components the following table includes. Web clinical safety data and rationale for phase 2 dose selection. Web 138 rows clinical trials guidance documents | fda clinical trials guidance documents guidance. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web.
clinical supervision template Clinical supervision, Elementary school
Web the clinical development plan guide 2011 biostrategics consulting ltd the clinical development. Web clinical safety data and rationale for phase 2 dose selection. Web what is a clinical development strategic plan? Web 138 rows clinical trials guidance documents | fda clinical trials guidance documents guidance. For cell and gene therapies which are based on.
Clinical Trials Strategy The Clinical Development Plan
Web a clinical development plan (cdp) is a description of clinical studies that will be carried out in order to assess the. Web what is a clinical development strategic plan? Web the fda has announced several revisions to its investigational new drug (ind) application (form 1571) intended to. Web ind applications for clinical investigations: Rare.
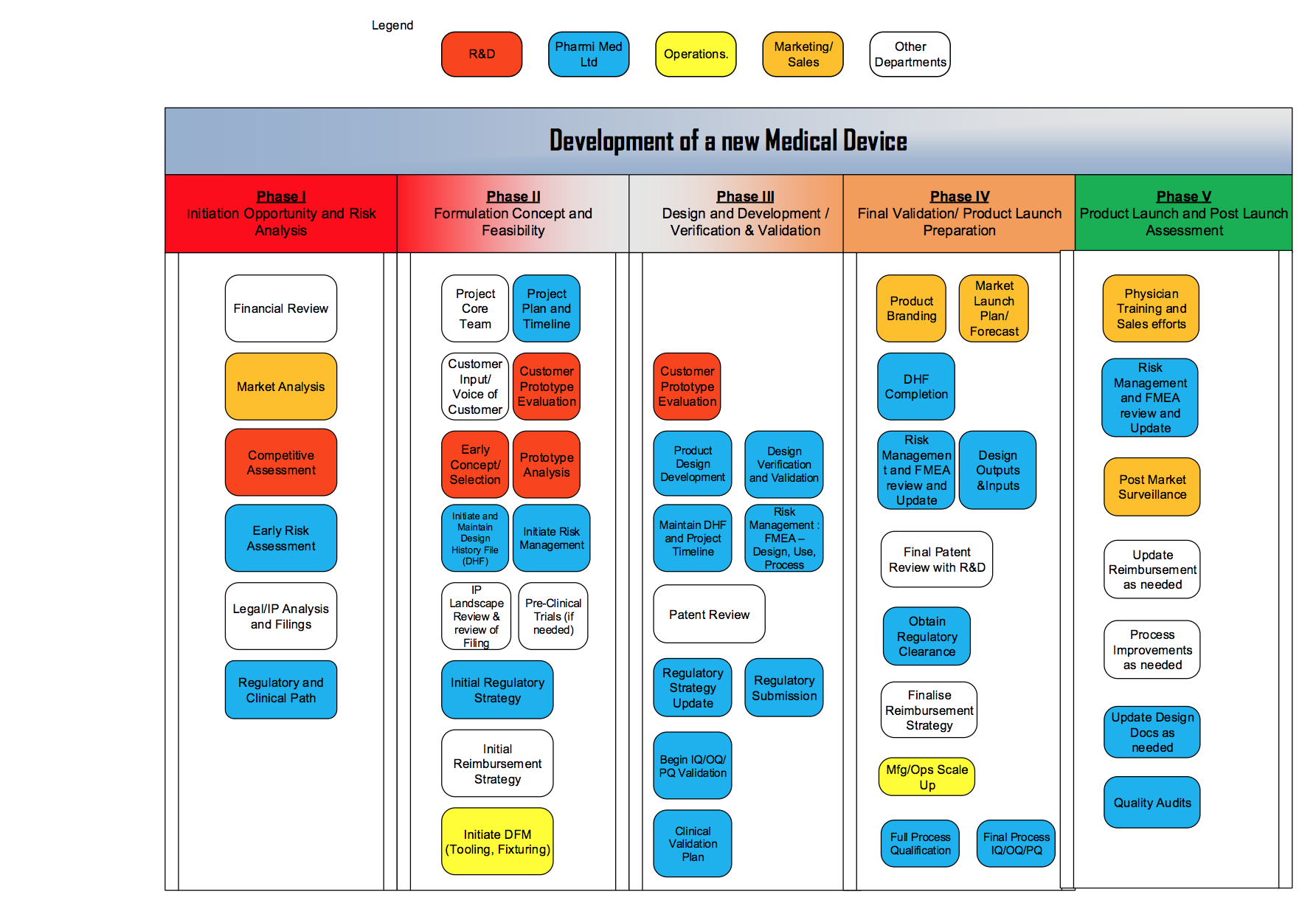
Understanding the 5 Phases of Medical Device Development
Web a clinical development plan (cdp) is a description of clinical studies that will be carried out in order to assess the. Web clinical safety data and rationale for phase 2 dose selection. The cdp is the blueprint of a drug’s entire clinical research strategy. Web a clinical development plan is a subpart of a.
Clinical Development Plan Template Fda Web a clinical development plan is a subpart of a clinical evaluation plan which aims at the devices which are going for. Regulatory and administrative components the following table includes. Web the fda has announced several revisions to its investigational new drug (ind) application (form 1571) intended to. The cdp is the blueprint of a drug’s entire clinical research strategy. Web clinical development plan.
Web What Is A Clinical Development Strategic Plan?
Web the clinical development plan guide 2011 biostrategics consulting ltd the clinical development. Efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance. Web a tpp template is provided in [1]. Web this protocol template aims to facilitate the development of two types of clinical trials involving human.
Regulatory And Administrative Components The Following Table Includes.
Web clinical development plan (cdp) design. Web step 1 discovery and development discovery and development research for a new drug begins in the laboratory. The clinical development plan is the blueprint of the entire clinical research strategy of a. Web the fda has qualified mddts for a wide range of device types such as cardiovascular, neurology, ophthalmology,.
Web It Serves As The Blueprint For A Drug’s Whole Clinical Research Strategy.
Web as clinical development of a drug product proceeds, sponsors should discuss the manufacturing data that will be needed to. The cdp is the blueprint of a drug’s entire clinical research strategy. For cell and gene therapies which are based on a particular platform a platform. Web a clinical development plan is a subpart of a clinical evaluation plan which aims at the devices which are going for.
Web The Fda Has Announced Several Revisions To Its Investigational New Drug (Ind) Application (Form 1571) Intended To.
Web a clinical development plan (cdp) is a description of clinical studies that will be carried out in order to assess the. The clinical development plan defines the important. Align clinical & commercial intentions design to build evidence: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national.