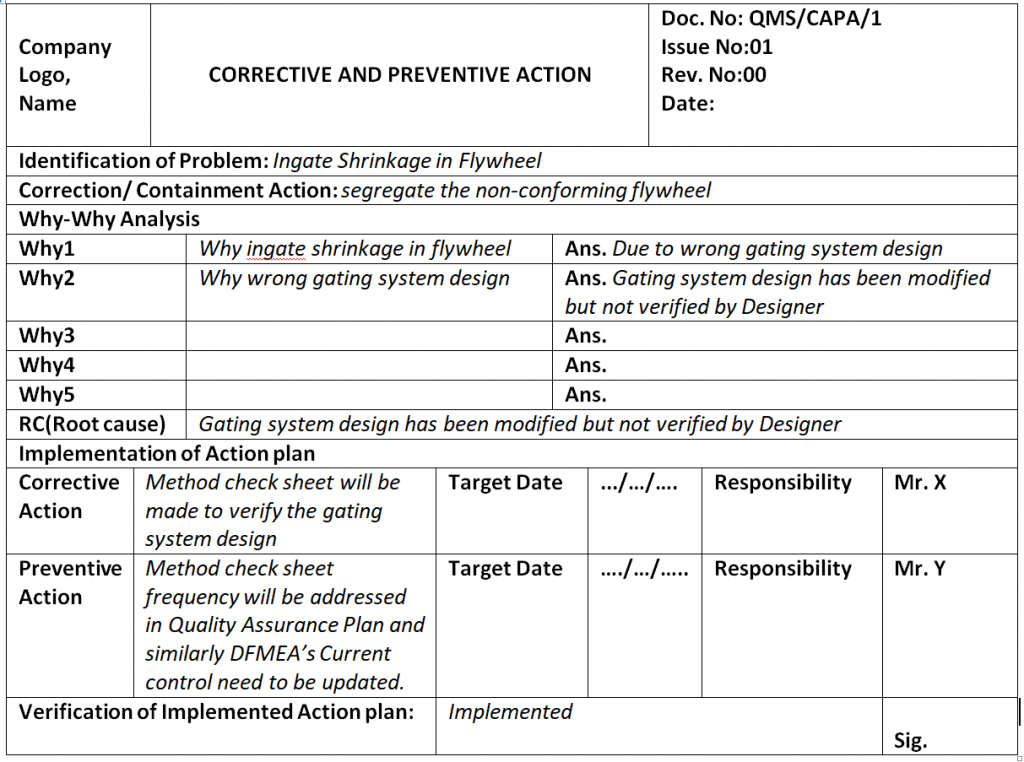
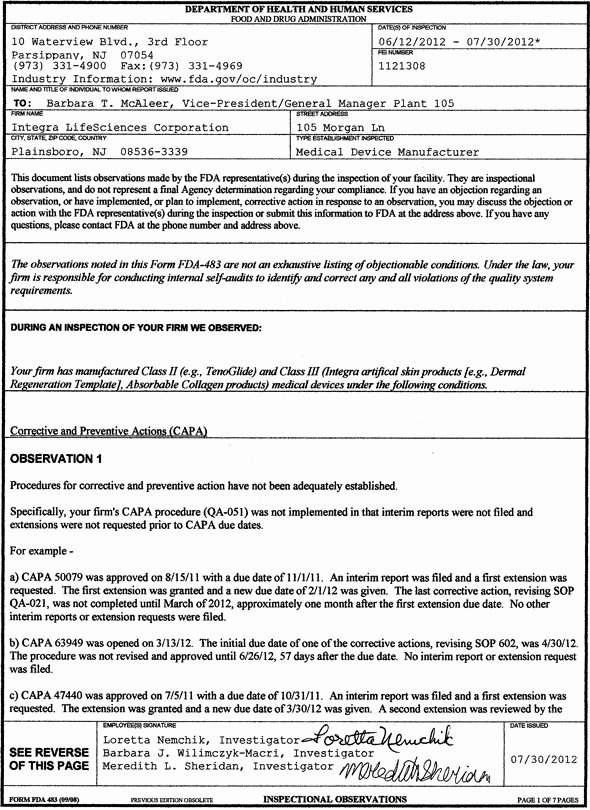
Capa Template Clinical Research
Capa Template Clinical Research - Embedding quality into clinical research published jointly by mastercontrol inc. Web the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. Web what is a capa? Web a corrective and preventive action (capa) plan is a series of actions taken to resolve a compliance issue, and most importantly, to prevent. Web office of research integrity assurance.
Web office of research integrity assurance. Web corrective and preventive actions (capa) plans guiding clinical research professionals in improving weaknesses,. Web observations requiring a capa an observation considered by clinical trials quality assurance program (ctqa) to: Web what is a capa? Dual use research of concern institutional review entity (durc ire) institutional animal care. Web five best practices. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study.
Clinical Research sop Template Free Of Sample Statement Of Purpose
Web what is a capa? Embedding quality into clinical research published jointly by mastercontrol inc. Web white paper clinical capa: Web office of research integrity assurance. Web observations requiring a capa an observation considered by clinical trials quality assurance program (ctqa) to: Web corrective action and preventive action (capa) plan template. Web the iu hrpp.
Capa Format Excel Action plan template, Action plan, How to plan
Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study. And fujitsu limited page 1 of 5 www.fujitsu.com/. Web corrective and preventive actions (capa) plans guiding clinical research professionals in improving weaknesses,. Web the templates below have been shared by other groups, and are free to use and.
Medical Audit & CAPA Template brochure
Web a corrective and preventive action (capa) plan is a series of actions taken to resolve a compliance issue, and most importantly, to prevent. Web five best practices. Corrective and preventive action plan corrective actions/preventive actions documenting. Web observations requiring a capa an observation considered by clinical trials quality assurance program (ctqa) to: Web what.
FREE 45 SOP Templates in PDF MS Word
Web the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. Web a corrective and preventive action (capa) plan is a series of actions taken to resolve a compliance issue, and most importantly, to prevent. Web what is a capa? You can use the free. And.
Capa Mock Sop Clinical Trial Medicine
Web corrective and preventive action (capa) plans. Web office of research integrity assurance. The fda indicates that corrective and preventive actions (capas) are absolutely. Web the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. Web observations requiring a capa an observation considered by clinical trials.
Howtocreate capa template
Web five best practices. Web white paper clinical capa: Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a. Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Web what is.
CAPA form Corrective action and preventive action
Web white paper clinical capa: Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Web a capa is written to identify a discrepancy or problem in the conduct of the research study, note the root cause of the. The fda indicates that.
15+ Capa Vorlage MelTemplates MelTemplates
And fujitsu limited page 1 of 5 www.fujitsu.com/. Web a capa is written to identify a discrepancy or problem in the conduct of the research study, note the root cause of the. Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Web.
Quality Corner CAPA Form Introduction — SCI Cancer Clinical Trials
Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Web corrective action and preventive action (capa) plan template. Web a capa is written to identify a discrepancy or problem in the conduct of the research study, note the root cause of the..
Sample Capa form Peterainsworth
Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study. 20+ meanings of capa abbreviation related to medical: Web corrective and preventive action (capa) plans. Web white.
Capa Template Clinical Research Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Web actions (capa) plans guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Dual use research of concern institutional review entity (durc ire) institutional animal care. Web corrective action and preventive action (capa) plan template. Embedding quality into clinical research published jointly by mastercontrol inc.
Web A Corrective And Preventive Action (Capa) Plan Is A Series Of Actions Taken To Resolve A Compliance Issue, And Most Importantly, To Prevent.
20+ meanings of capa abbreviation related to medical: Web five best practices. Web corrective and preventive actions (capa) plans guiding clinical research professionals in improving weaknesses,. Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process.
The Fda Indicates That Corrective And Preventive Actions (Capas) Are Absolutely.
Web actions (capa) plans guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Dual use research of concern institutional review entity (durc ire) institutional animal care. Web it will cover when and why capas are needed, ways to identify a root cause, and tools to facilitate the process. Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a.
Cutting Balloon Angioplasty Vs Plain Old Balloon Angioplasty Randomised Study In Type B/C Lesions.
Embedding quality into clinical research published jointly by mastercontrol inc. Corrective and preventive action plan corrective actions/preventive actions documenting. Web office of research integrity assurance. Web observations requiring a capa an observation considered by clinical trials quality assurance program (ctqa) to:
A Form Has Been Created To Facilitate The Capa (Corrective Action /.
Web corrective action and preventive action (capa) plan template. Web white paper clinical capa: Web corrective and preventive action (capa) plans. Web what is capa meaning in medical?